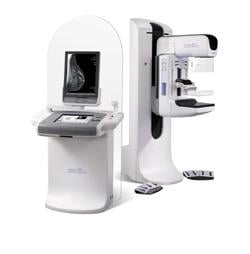
June 20, 2011 – The Selenia Dimensions 2-D/3-D mammography system won a gold Medical Design Excellence Award (MDEA) at the Medical Design and Manufacturing East 2011 Conference and Exposition. Selenia is manufactured by Hologic.
The Medical Design Excellence Award recognizes Hologic for innovation in design and engineering for Selenia Dimensions, the first commercially available tomosynthesis system for breast cancer screening and diagnosis. Unlike prior-generation mammography systems, which generate two-dimensional (2-D) images, a breast tomosynthesis system produces three-dimensional (3-D) images, which are intended to reveal the inner architecture of the breast, free from the distortion typically caused by tissue shadowing or density.
The Hologic system gives radiologists the option of offering their patients a conventional 2-D digital mammogram and a 3-D tomosynthesis exam – all in one compression, in just seconds.
In clinical studies Hologic submitted to the U.S. Food and Drug Administration (FDA), radiologists reading 2-D mammography plus 3-D breast tomosynthesis images compared to 2-D images alone demonstrated superior clinical performance in specificity, the confidence to rule out breast cancer without recalling the patient for further study, and also demonstrated improved sensitivity, the proportion of mammograms which include breast cancers that were correctly diagnosed.
In accepting the award for Hologic, Ian Shaw, director of breast health advanced product development, noted, "The Medical Design Excellence Award is welcome recognition for the achievements of the many people behind the scenes who are responsible for developing the Selenia Dimensions mammography system." Farm Design Inc., a New England medical product development company, assisted Hologic with the industrial design, human factors, engineering and user interface of the product.
Hologic's 2-D/3-D breast tomosynthesis systems are available for sale worldwide. Selenia Dimensions 3D tomosynthesis was approved for sale in the United States by the FDA on Feb. 11, 2011.
This is the fourth time a Hologic product has won a top award in the MDEA program. The Company's Sentinelle Vanguard breast imaging and interventional coil won gold in 2010. The Sentinelle breast imaging coil transforms a Magnetic Resonance Imaging (MRI) scanner into a powerful breast imaging and interventional tool. Hologic's ThinPrep imaging system won gold in 2004. The ThinPrep imaging system is a fully integrated, interactive computer system that assists cytotechnologists and cytopathologists in the primary screening and diagnosis of cervical cancer using ThinPrep Pap test slides. The MammoSite radiation therapy system won gold in 2003. The MammoSite catheter and balloon assembly delivers radiation from within the tumor-resected cavity after breast conservation therapy. MammoSite targeted radiation therapy works from the inside, meaning that a higher daily dose can be used for a shorter period of time – five days versus five to seven weeks.
For more information: www.hologic.com


 February 17, 2026
February 17, 2026 









