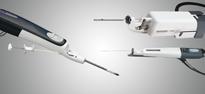
December 22, 2010 – The Mammotome molecular imaging (MI) system, which is used to conduct minimally invasive, vacuum-assisted breast biopsy (VABB) with molecular imaging guidance, has received 510(k) clearance. It is the first device of its kind to receive FDA clearance, and it is commercially available to healthcare facilities specializing in breast cancer screening and diagnosis.
The Mammotome biopsy system has been available for use with traditional imaging modalities, including stereotactic (X-ray), ultrasound and magnetic resonance imaging (MRI). The new Mammotome MI system facilitates minimally invasive breast biopsy guided by molecular imaging techniques, such as positron emission mammography (PEM) and breast-specific gamma imaging (BSGI) to localize lesions.
In traditional screening or diagnostic imaging of the breast, clinicians look at the breast for anatomical changes. With molecular imaging, however, the clinicians look for unusual metabolic activity in the breast. This is accomplished by injecting the patient with a small amount of radiopharmaceutical tracer. These radioactive substances circulate and get trapped in abnormal cells creating a unique “hot spot” which can be imaged and biopsied with the Mammotome MI system.
A procedure with the Mammotome biopsy system involves a single insertion of a needle equipped with a vacuum system into a tiny 1/4-inch incision. The system gently vacuums, cuts and removes suspicious breast tissue for analysis and diagnosis. Since the incision is so small, no stitches are required. Instead, only an adhesive bandage is needed, and most patients can return to normal activity immediately following the procedure. The outpatient procedure typically takes less than an hour, and scarring is minimal.
For more information: www.mammotome.com or www.devicormedical.com


 March 06, 2026
March 06, 2026 









