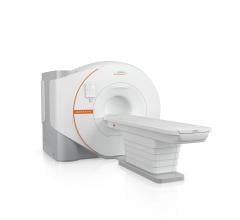
July 2, 2025 — Philips has received FDA 510(k) clearance for SmartSpeed Precise1 MR’s latest deep learning ...
FDA
June 26, 2025 — Siemens Healthineers has received Food and Drug Administration clearance for the Magnetom Flow.Ace, its ...
Jun. 24, 2025 — GE HealthCare has announced that the U.S. Food and Drug Administration (FDA) approved an updated label ...
SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
June 24, 2025 —Smart Soft Healthcare has announced that CoLumbo, the company's advanced AI spine assistant, has received ...
June 17, 2025 — Bayer has announced the submission of a New Drug Application (NDA) to the US Food and Drug ...
June 17, 2025 — Royal Philips has announced the global launch of the Flash Ultrasound System 5100 POC — a new point-of ...
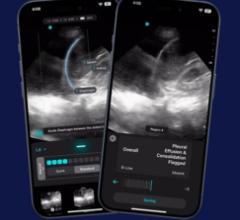
June 18, 2025 — Exo recently announced that now included on its Exo Iris is the first ever FDA 510(k) cleared AI for ...
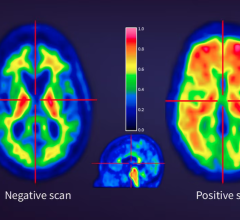
June 2, 2025 — Clairity, Inc., a digital health innovator advancing AI-driven healthcare solutions, has received U.S ...
May 29, 2025 — Hyperfine, Inc., producer of the world’s first FDA-cleared AI-powered portable MRI system for the brain — ...
May 13, 2025-- GE HealthCare recently announced the U.S. Food and Drug Administration (FDA) has approved a pediatric ...
May 19, 2025 - Arineta, a provider of cardiovascular imaging solutions, recently announced that its SpotLight Duo ...
May 15, 2025 — GE HealthCare has launched CleaRecon DL, technology powered by a deep-learning algorithm, to improve the ...
May 12, 2025 — GE HealthCare recently unveiled Signa Sprint, an FDA 510(k) pending[1] ultra-premium wide bore 1.5T high ...
May 5, 2025 — GE HealthCare recently announced that the U.S. Food and Drug Administration (FDA) granted 510(k) clearance ...
May 2, 2025 — GE HealthCare has announced an intended expansion of its radiation oncology portfolio as well as the ...
April 17, 2025 — Nano-X Imaging LTD has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for ...
April 15, 2025 — CureMetrix, a provider of AI-driven medical imaging solutions, has announced that its AI-based cmAngio ...
April 7, 2025 — DOSIsoft, a provider of patient-specific imaging and dosimetry software solutions, recently announced ...
March 28, 2025 — Novartis has announced that the US Food and Drug Administration (FDA) approved Pluvicto (lutetium Lu ...
March 20, 2025 — GE HealthCare has launched Invenia Automated Breast Ultrasound (ABUS) Premium, the latest 3D ultrasound ...


 July 03, 2025
July 03, 2025