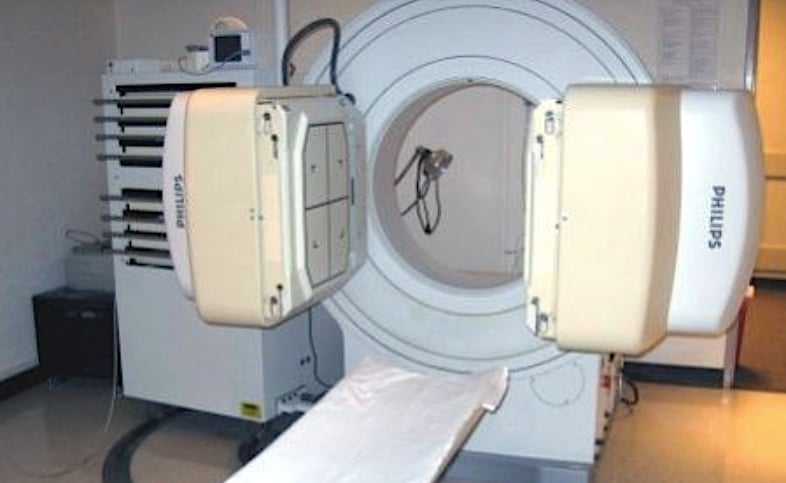
Philips Medical System is recalling its older Forte Gamma Camera SPECT imaging systems due to the possibility of the detectors falling off of the unit onto the patient. The two gamma cameras can be seen in this photo on either side of the patient bed. These can be rotated above the patient.
November 5, 2019 — Philips Medical System is recalling the Forte Gamma Camera System due to the potential for the 660-pound detector to detach from the device without warning. The vendor said this could result in a serious injury, such as crushing, trapping or killing the patient. The U.S. Food and Drug Administration (FDA) reported Philips received one customer complaint, but no serious injuries or deaths were reported.
The FDA said this is a Class I recall, due to potential for the nuclear imaging detector to become detached, which can result in injury or death. Philips told its customers to discontinue use of the Forte Gamma Camera System until further notice. Philips initiated its recall Sept. 19, 2019. It includes 852 devices recalled in the United States.
The FDA and Philips are taking precautions because of a June 2013 accident, when the detector on a GE Healthcare SPECT system fell onto a patient and killed them during a scan at the James J. Peters VA Medical Center in the Bronx, N.Y. That incident resulted in a similar recall at the time for GE's nuclear imaging systems. Read the article Patient Killed During Nuclear Imaging Scan.
The Forte Gamma Camera System is an older single photon emission computed tomography (SPECT) nuclear imaging system. These systems were manufactured between Jan. 1, 1998 and Dec. 31, 2008. It is used to create images of structures or functions inside the body of patients using a radioisotope tracer to diagnose, plan treatment and evaluate many conditions, including cancer and cardiac perfusion.
The recalled products include the following imaging system:
• Philips Medical System Forte Gamma Camera System
• Forte (882020)
• Forte Jetstream (882290)
• Forte Jetstream upgrade (882291)
• Forte Jetstream AZ (882320)
• Forte Jetstream AZ upgrade (882321)
• Diamond Select Forte (889456)
• Diamond Select Forte JETStream (889471)
A portion of the gamma camera head weight comes from the heavy lead collimators built into them.
Philips sent a letter to customers who purchased the affected systems and provided the following instructions:
• Discontinue use of the Forte Gamma Camera System until further notice
• Maintain the letter with the Forte Gamma Camera System until a correction is made to the system
• Complete the Customer Response Form and e-mail it to [email protected]
Healthcare providers and patients are encouraged to report adverse events or side effects related to the use of these products to the FDA's MedWatch Safety Information and Adverse Event Reporting Program. Complete and submit the report online. Download form or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the form, or submit by fax to 1-800-FDA-0178.


 February 03, 2026
February 03, 2026