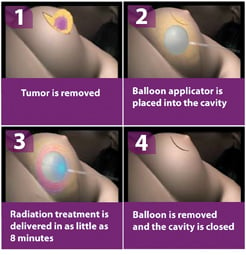
Intraoperative Radiation Therapy (IORT) procedure in four steps.
The current standard of care for treatment of early stage breast cancer is mastectomy or lumpectomy followed by five to seven weeks of daily whole breast radiation therapy (WBRT) treatments. Post-operative accelerated partial breast irradiation (APBI) is slowly replacing whole breast external beam irradiation as an alternative in treatment of early stage lymph node-negative breast cancer. This was originally performed as limited-field breast brachytherapy using multiple interstitial catheters placed post-operatively around the lumpectomy site. Radioactive interstitial wire implants were then placed into the catheters.
More recently, APBI has been delivered as post-operative intensity-modulated radiotherapy, 3-D conformal radiotherapy or balloon catheter radiotherapy. APBI is usually delivered to the lumpectomy site in 10 post-operative fractions given twice a day, six hours apart, throughout the course of five days. Results to date from all four techniques are favorable, showing good cosmesis and recurrence rates comparable to standard whole breast irradiation.
Trial Studies
Intraoperative radiation therapy (IORT) allows for delivery of a single fraction of radiation to the lumpectomy site while still in the operating room. The use of IORT has been reported in two European studies. The ELIOT trial, published by Veronesi in 2008, reported the results of 1,246 patients treated with intraoperative electron beam radiotherapy using the Sordina IORT Technologies’ Novac 7 and Liac devices.1 Five-year survival was roughly calculated to be 96.5 percent. New primary carcinoma diagnosed in other breast quadrants was 1.3 percent. Four-year follow-up data for another trial, the TARGIT-A trial, was published by Vaiyda in 2010. The Zeiss Intrabeam device was used to treat 1,232 patients.2 Results were considered equivalent to lumpectomy followed by five weeks of standard whole breast irradiation. Five-year follow-up TARGIT-A trial data has been presented for 1,222 patients, showing that IORT still meets non-inferiority criteria when compared to WBRT.3 IORT is now also available using a disposable balloon applicator device as part of the Xoft electronic brachytherapy system. Results of 11 patients receiving a single 20 Gy intraoperative fraction were reported by Ivanov in 2010.4 Mean follow-up was for 12 months. There were no cancer recurrences in this short follow-up time interval. The results of 40 patients using the Xoft System were presented in 2013.5 There were no recurrences after a mean follow-up of 15 months.
Reduced Treatment Time
Intraoperative radiation therapy using electronic brachytherapy for treatment of early stage breast cancer is currently under investigation. Electronic brachytherapy is delivered using portable equipment that runs conventional electricity over a tungsten or gold crystal to produce radiation. The resultant radiation has a short penetration, allowing for use in the operating room. Leaded bunkered rooms are no longer necessary. The personnel and patients are protected by standard leaded drapes and portable shields. The balloon applicator is placed into the lumpectomy cavity and then attached to a controller.
Doses, which are individually calculated, are dependent on the size and conformation of the lumpectomy cavity. Most IORT clinical trials include women with a single focus of biopsy-proven invasive ductal carcinoma who have cancer-free lymph nodes. Some trials include women who have been diagnosed with ductal carcinoma in situ (DCIS). If enrolled, patients undergo a standard sentinel lymph node (SLN) analysis and lumpectomy with intraoperative margin evaluation. The SLNs must be cancer-free. Adequate margins are determined radiographically and on gross examination by the pathologist. If cleared by these parameters, a shield is placed against the chest wall to protect the underlying organs. A balloon applicator attached to the Xoft System is placed into the lumpectomy cavity. Intraoperative ultrasound is used to document good apposition of surrounding breast tissue and an acceptable distance between skin and applicator surface. Patients receive a single fraction of electronic brachytherapy through the applicator of 20 Gy. The applicator and shield are then removed and the operation is completed using current standards of care. Final pathology confirms adequate margins and that the SLNs are cancer-free. Additional treatment recommendations follow local medical standards.
IORT is well tolerated by patients and is usually performed in an outpatient setting. Patients are pleased both with the cosmetic outcome and convenience of the procedure. The cosmetic results are favorable as the radiation is localized to only the lumpectomy site. They are no longer inconvenienced by a five- to seven-week schedule of daily post-operative whole breast irradiation. It is predicted that this will reduce treatment costs.
Promising Alternative
Intraoperative radiation treatment using electronic brachytherapy has shown promise as an alternative to other forms of APBI or whole breast irradiation in the treatment of patients with early stage node negative breast cancer. Clinical trials documenting long-term outcomes are in progress.
References
1. Veronesi U, Orecchia R, Luini A, et al. “Full dose Intraoperative radiotherapy with electrons (ELIOT) during breast conserving surgery. Experience with 1246 cases.” ECancer Medical Science. Volume: 1 Article Number: 65, 2008.
2. Vaidya JS, Joseph DJ, Tobias JS, et al. “Targeted intraoperative
radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomized,
non-inferiority phase 3 trial.” Lancet 2010; 376 (9735), 91-102.
3. Vaidja JS. San Antonio Breast Cancer Symposium, Dec. 4-8, 2012.
4. Ivanov O, Dickler A, Lum BY, et al. “Twelve-month follow-up results of a trial utilizing Axxent electronic brachytherapy to deliver intraoperative radiation therapy for early-stage breast cancer.” Ann Surg Oncol 2011;18(2): 453-458.
5. Schwartzberg, BS. American Society of Breast Surgeons 14th Annual Meeting, Chicago, May 1-5, 2013.
Barbara Schwartzberg, M.D., is a private practice breast surgeon at Rose Medical Center in Denver, Colo. She received her B.A. in chemistry from Franklin and Marshall College, her M.S. in pharmaceutical chemistry from Purdue University and her M.D. from George Washington University before doing her general surgery residency at the University of Colorado. She enjoys researching new technologies that allow for easier, more targeted treatment of early stage breast cancer.


 February 18, 2026
February 18, 2026 









