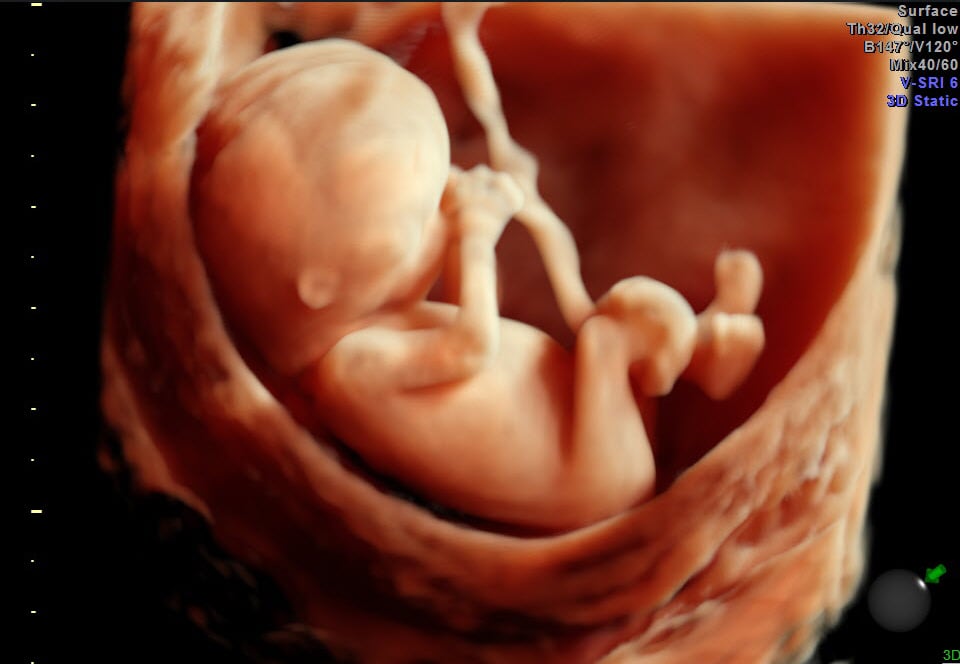
December 17, 2014 — The U.S. Food and Drug Administration (FDA) has put out a public safety notice asking expectant parents not to use ultrasound systems without a medical need.
Fetal ultrasound imaging provides real-time images of the fetus. Doppler fetal ultrasound heartbeat monitors are hand-held ultrasound devices that let you listen to the heartbeat of the fetus. Both are prescription devices designed for use by trained healthcare professionals. They are not intended for over-the-counter (OTC) sale or use, and the FDA strongly discourages their use for creating fetal keepsake images and videos.
"Although there is a lack of evidence of any harm due to ultrasound imaging and heartbeat monitors, prudent use of these devices by trained healthcare providers is important," says Shahram Vaezy, Ph.D., an FDA biomedical engineer. "Ultrasound can heat tissues slightly, and in some cases, it can also produce very small bubbles (cavitation) in some tissues."
The long-term effects of tissue heating and cavitation are not known. Therefore, ultrasound scans should be done only when there is a medical need, based on a prescription, and performed by appropriately trained operators.
Fetal keepsake videos are controversial because there is no medical benefit gained from exposing the fetus to ultrasound. FDA is aware of several enterprises in the United States that are commercializing ultrasonic imaging by making fetal keepsake videos. In some cases, the ultrasound machine may be used for as long as an hour to get a video of the fetus.
While FDA recognizes that fetal imaging can promote bonding between the parents and the unborn baby, such opportunities are routinely provided during prenatal care. In creating fetal keepsake videos, there is no control on how long a single imaging session will last, how many sessions will take place, or whether the ultrasound systems will be operated properly. By contrast, Veazy says, “Proper use of ultrasound equipment pursuant to a prescription ensures that pregnant women will receive professional care that contributes to their health and to the health of their babies.”
Similar concerns surround the OTC sale and use of Doppler ultrasound heartbeat monitors. These devices, which are used for listening to the heartbeat of a fetus, are legally marketed as "prescription devices," and should only be used by, or under the supervision of, a health care professional.
For more information: www.fda.gov


 February 18, 2026
February 18, 2026 









