September 14, 2012 — A radiologist’s ability to detect pneumonia early — so it can be treated quickly — is improved by Riverain Technologies software that suppresses the ribs and clavicles on a chest X-ray, suggests new research published online by European Radiology.
This week at the American Telemedicine Association’s 2012 Fall Forum, Medweb introduced several new products designed to present first responders and clinicians with immediate access to tools for use in the field and to save precious time in emergency situations, providing new opportunities to improve both overall patient treatment and clinical outcomes.
September 14, 2012 — IBA Molecular North America Inc., a manufacturer and distributor of PET imaging tracers, has announced the introduction of its Centers for Innovation, two unique facilities designed for advanced radiopharmaceutical contract development and manufacturing. The centers are located in Totowa and Somerset, N.J. Complementing these sites are IBA Molecular's production capabilities for isotopes such as I-124, F-18, Zr-89 and in the future, Cu-64 and Y-86. The centers are expected to play an important role in the development of a new generation of disease-specific tracers and companion theragnostics.
Radiology departments have many different needs and face a wide variety of challenges that can impact their departments ...
Calgary Scientific Inc. introduced ResolutionMD 3.1, a Web and mobile universal medical image viewing software that now supports 10 languages.
Barco announced the launch of its MDSC-2226, a 26-inch surgical display providing full compatibility with Barco’s networked digital operating room. Featuring an integrated IP-to-AV decoder, an easy-clean design and a unique cable management system, the MDSC-2226 is a perfect fit for modern digital operating rooms.
Biodex Medical Systems Inc. has announced the availability of a new C-arm tabletop design for Surgical C-Arm Tables – 840, 846 and Pain Management C-Arm Table – 870. These tables are now offered with the option of two styles of tabletop to choose from. The new rectangular tabletop design offers additional space to allow for even image quality for long leg runoff studies. The classic contoured tabletop design allows C-arm positioning for the closest possible patient access, achieving optimum image resolution.
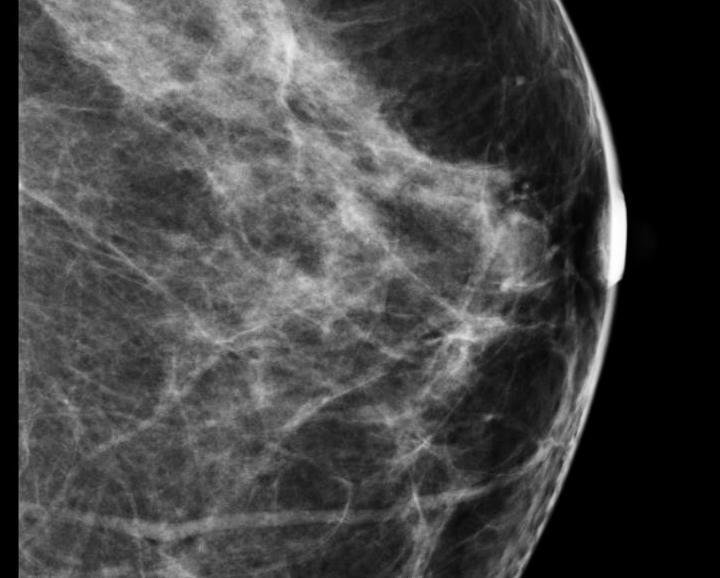
Despite decades of progress in breast imaging, one challenge continues to test even the most skilled radiologists ...
September 14, 2012 — The U.S. Food and Drug Administration (FDA) has approved the production and use of Choline C-11 injection, a positron emission tomography (PET) imaging agent used to help detect recurrent prostate cancer. Choline C-11 injection is administered intravenously to produce an image that helps to locate specific body sites for follow-up tissue sampling and testing in men with recurrent prostate cancer.
September 14, 2012 — DR Systems announced that its cloud-based ambulatory electronic health record (EHR) for radiologists, the e|HR Meaningful Use for Medical Imaging solution, is now commercially available after successfully completing beta testing.
September 12, 2012 – Positron Corp. announced that Jubilant DraxImage Inc., a Jubilant Life Sciences Company, and Positron have executed a Letter of Intent pertaining to Positron's supply of Active Pharmaceutical Ingredient (API) grade Sr-82 for the JDI Sr-82/Rb-82 generator; JDI's and Positron's co-promotion of JDI Sr-82/Rb-82 generators to end-users (upon FDA clearance); and Positron's lifecycle management for expired JDI Sr-82/Rb-82 generators.
Bayer Radiology’s Barbara Ruhland and Thom Kinst discuss how radiology departments can address the many different ...
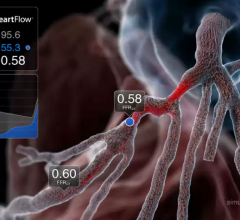
September 11, 2012 — Data presented at the 2012 ESC Congress in Munich from the prospective DeFACTO study show that, when compared to standard coronary angiography, the noninvasive assessment of fractional flow reserve by computed tomography (FFR-CT) provides a more accurate determination of which lesions require invasive evaluation.
Sept. 10, 2012 – A mammography and women’s health advocacy group, Are You Dense Inc., blasted the American College of Radiology (ACR) and the Society of Breast Imaging (SBI) for their positions on recent research published in the Journal of the National Cancer Institute. The article detailed the risk of death by stage of cancer for women with breast cancer, which Are You Dense said is misleading in its presentation of data to the public.
Sept. 10, 2012 — High mammographic breast density, which is a marker of increased risk of developing breast cancer, does not seem to increase the risk of death among breast cancer patients, according to a study led by Gretchen L. Gierach, Ph.D., of the National Cancer Institute (NCI), part of the National Institutes of Health. The research was conducted in collaboration with investigators from the NCI-sponsored Breast Cancer Surveillance Consortium (BCSC).
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
September 10, 2012 — TeraRecon announced the launch of iNtuition Review, iNtuition Enterprise Medical Viewer (iEMV) and iNtuition Share. The software suite enhances the iNtuition portfolio with multimodality review solutions for advanced imaging, such as cardiac and breast procedures, with enterprise distribution and image sharing capability.
September 10, 2012 — In a recent study, researchers found that coffee consumption is inversely related to mortality, with consumption of greater than or equal to six cups of coffee per day associated with a 10 percent decreased mortality rate in men and 15 percent decreased mortality in women.
September 7, 2012 — RaySearch Laboratories AB announces that version 3.0 of RaySearch’s RayStation treatment planning system has been released for clinical use in the European Union, United States, Japan and Australia, and is pending regulatory approval in Canada, China, New Zealand and South Korea.
September 7, 2012 — The American Medical Association (AMA) recently sent letters to Sen. Ben Cardin of Maryland and Rep. Pete Olson of Texas supporting passage of the Diagnostic Imaging Services Access Protection Act (H.R. 3269/S. 2347).
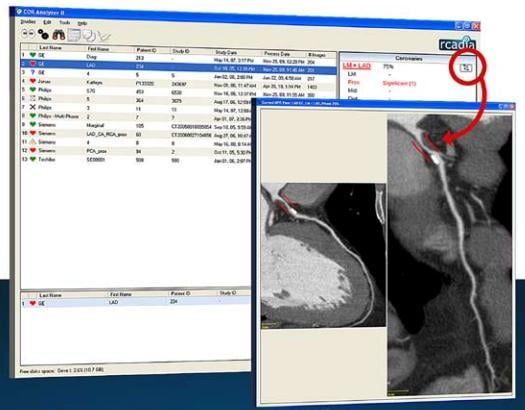
There has been much debate whether computer-aided detection software for computed tomography (CT) scanners to detect coronary blockages is really a worthwhile expense. There has long been apprehension by experienced cardiologists and radiologists who can easily read coronary CT angiography (CCTA) scans for plaque burden. However, the technology may have a niche application for immediate STAT reads of chest pain patients during off-hours, especially at rural medical centers.
September 6, 2012 — The American Society of Radiologic Technologists (ASRT) launched its new online breast imaging educational series, Breast Imaging Basics: The Series. The 10-module interactive series provides medical imaging professionals and students with a comprehensive overview of the fundamentals of breast imaging.
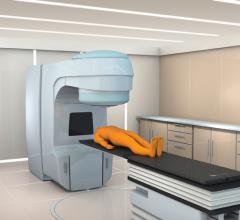
As radiation treatments have advanced in complexity, cancer treatment facilities have faced greater demands for accuracy and precision in treatment. Northwestern Lake Forest Hospital recently installed CIVCO Medical Solutions’ Protura Robotic Patient Positioning System at its Center for Advanced Radiation Medicine in Lake Forest, Ill.
Twenty-seven radiologists at Washington Radiology Associates (WRA), a large private imaging practice in the Washington, D.C., metropolitan area, perform 85,000 mammograms annually at six clinical offices. In 2011, WRA replaced all of its 2D mammography systems with 15 Hologic Selenia Dimensions systems.


 September 14, 2012
September 14, 2012