In addition to demonstrating their speed, agility and strength at the 2018 National Football League (NFL) Scouting Combine, top college football players also undergo comprehensive physical evaluations that include X-ray exams. This year a Carestream DRX Core detector is being used with the existing X-ray system at Lucas Oil Stadium (Indianapolis) to produce high-quality diagnostic images in seconds.
The University of Texas MD Anderson Cancer Center and RaySearch Laboratories announced a new strategic alliance with the aim of enhancing cancer radiation therapy through several initiatives. The partnership will focus on goals including more precisely targeting tumors and improving upon, and making more available, adaptive radiation therapy (ART), currently only used at highly specialized care centers.
The U.S. Food and Drug Administration (FDA), Centers for Disease Control and Prevention (CDC) and American Society for Microbiology (ASM), along with other endoscope culturing experts, announced the availability of voluntary, standardized duodenoscope surveillance sampling and culturing protocols. Hospitals and healthcare facilities that utilize duodenoscopes can, in addition to meticulously following manufacturer reprocessing instructions, take these additional steps to further reduce the risk of infection and increase the safety of these medical devices. These protocols are an update to the Interim Duodenoscope Surveillance Protocol released by CDC in March 2015.
Radiology departments have many different needs and face a wide variety of challenges that can impact their departments ...
February 26, 2018 – The American Society of Echocardiography (ASE) and its International Alliance Partners are joining ...
Visage Imaging Inc. announced the latest version of the Visage 7 Enterprise Imaging Platform, Visage 7.1.11, has been released for global availability. Visage 7 enables enterprise imaging with fast, thin-client, server-side processing technology, as well as simple diagnostic mobile access via Visage Ease Pro.
February 23, 2018 — A portable ultrasound can help nephrologists better detect fluid in the lungs of patients with end ...
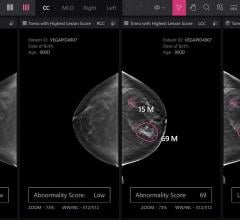
Despite decades of progress in breast imaging, one challenge continues to test even the most skilled radiologists ...
Qioptiq, an Excelitas Technologies Company, recently introduced SlimLine for X-ray Diagnostics featuring Spine Mode. The new operation mode enables automatic optimization of overexposed images for maximum contrast and visibility of the spine.
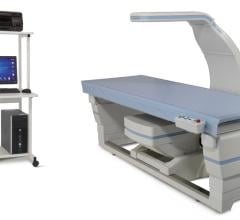
Hologic Inc. announced it has signed an agreement with the University of Minnesota to be the exclusive provider of Dexalytics: TEAMS in North America. The partnership provides the sports science and human performance industry with a solution to harness body composition data obtained from dual X-ray absorptiometry (DXA) scans, compare it to sport- and position-specific standards, and provide actionable information that can help collegiate and professional trainers, coaches and medical staff train better athletes.
The RamSoft team will showcase radiology solutions to help users cut costs and save time at the 2018 Healthcare Information and Management Systems Society (HIMSS) conference, March 5-9 in Las Vegas. Offerings will include RamSoft PowerServer RIS/PACS (radiology information system/picture archiving and communication system), a radiology system that enables healthcare practices to operate diagnostic imaging more efficiently. The RamSoft team will show how PowerServer RIS/PACS allows users to customize workflow to meet their needs; empowering customers to increase efficiency and grow their practices.
Bayer Radiology’s Barbara Ruhland and Thom Kinst discuss how radiology departments can address the many different ...
February 22, 2018 — U.K.-based medical imaging software provider Mirada Medical has released DLCExpert, the first ...
FUJIFILM Medical Systems U.S.A., Inc. and FUJIFILM SonoSite Inc., are offering a full-suite pediatric solutions portfolio, complete with digital radiography (DR), healthcare IT and point-of-care ultrasound to serve the world's most unique patients.
The European Congress of Radiology (ECR) announced the theme of its 2018 annual meeting, Feb. 28-March 4 in Vienna Austria, will be “Diverse & United,” focusing on the specialty’s diversity and will promote science and innovation.
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
This 5 megapixel, high-brightness color monitor has the high-definition display necessary for breast imaging. It ...
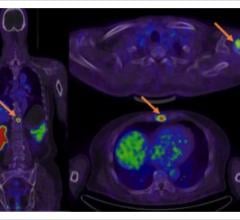
Blue Earth Diagnostics announced that Axumin (fluciclovine F 18) injection has been added to the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology for Prostate Cancer (Version 1.2018). These updated NCCN Guidelines state that F-18 fluciclovine positron emission tomography/computed tomography (PET/CT) or PET/magnetic resonance imaging (MRI) should be considered in the clinical workup of patients with recurrence or progression of their prostate cancer. This recommendation is based on uniform NCCN consensus that the intervention is appropriate.
Barco will showcase its full line of radiology and mammography display systems with online quality assurance (QA) software at the 2018 Healthcare Information and Management Systems Society (HIMSS) conference, March 5-9 in Las Vegas. The company will focus on how its solutions can help boost diagnostic accuracy, streamline operations and reduce costs.
At the 2018 annual meeting of the Healthcare Information and Management Systems Society (HIMSS), March 5-9 in Las Vegas, Konica Minolta Healthcare Americas Inc. will demonstrate the infusion of intelligent analytics into its Exa Enterprise Imaging platform.The analytics package will enable customers to boost department productivity, maximize asset utilization and enhance the patient experience.
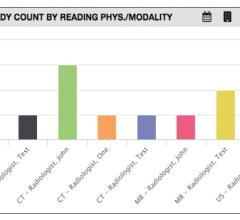
Deep learning startup company Aidoc announced what it calls the world’s first and only comprehensive, full-body solution utilizing artificial intelligence (AI) to help analyze computed tomography (CT) scans, highlighting medical findings for radiologists. The workflow-integrated solution offers support for radiologists covering areas such as the head, c-spine, chest and abdomen.
February 20, 2018 – Digital transformation is happening in all industries, and it will happen in healthcare whether ...
February 20, 2018 – LUMEDX Corporation, a top cardiovascular data intelligence company, will show off the latest in ...


 February 27, 2018
February 27, 2018