A new brush-free scrubbing product, Surgicept Waterless Surgical Hand Antiseptic is designed to provide rapid and ...
Designed for fast, accurate and heavy-capacity patient weighing, the Detecto IB600 scale features a flame retardant ...
A complete solution for tracking assets and people within hospitals, HealthTrax includes RFID asset tags and enterprise ...
Radiology departments have many different needs and face a wide variety of challenges that can impact their departments ...
Designed to help streamline the charting of vital signs data into the EMR, the integriti vital signs device collects ...
The new P10-4 transducer is designed for imaging neonatal, infant and pediatric patients. The new P4-1 small footprint ...
A Web-native IT solution, Sorian Community Access, provides single sign-on access to browser and non-browser-based ...
Despite decades of progress in breast imaging, one challenge continues to test even the most skilled radiologists ...
American Mammographics has developed the new S.O.F.T. Paddle for Screening Mammography. S.O.F.T. Paddle is the "Special ...
Candelis’ ImageGrid industry-standard DICOM architecture provides 1 to 6 terabytes, with optional expansion to 38 ...
NeuroLogica Corp. develops, manufactures and markets innovative medical imaging equipment for healthcare facilities and ...
Bayer Radiology’s Barbara Ruhland and Thom Kinst discuss how radiology departments can address the many different ...
The 3MP monochrome LCD monitor for PACS, chest radiology, CT, MRI and angiography has been rolled out. RadiForce G33, a ...
The Aplio XV now offers Expanded Differential Tissue Harmonics, a patented, proprietary harmonic technique especially ...
IMPAX Enterprise, an IT solution that addresses the intricate and often complex enterprise-wide issues for hospitals and healthcare facilities, has been launched globally. The solution provides an integrated image and information management approach to those issues.
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
Bar coding for all prescription and over-the-counter medications — which are commonly used and dispensed in the acute ...
Virtua is an integrated disc recorder, and is a complete disc publishing solution for recording and labeling CD and DVD ...
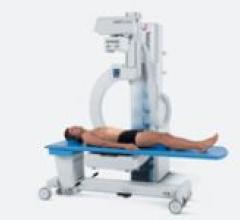
The newest C-arm system, ddRFormula, features numerous technological innovations in a compact design. It incorporates ...
The 4D Localization System is a target localization platform based on detection of AC electromagnetic markers, called Beacon transponders. Beacon transponders send signals to the 4D localization system that generate objective location instructions to the radiation therapist to register the patients treatment target to isocenter prior to treatment.
AccessRAD, an integrated RIS/PACS solution, is designed to meet the needs of acute care hospitals, enterprise-wide ...
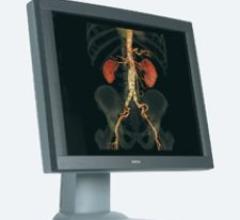
NIO COLOR 2MP from Barco features a 2-megapixel (1,600 by 1,200 resolution), 20.1-inch color LCD, offering superior ...
The InDex archiving service will be enhanced with a new Tier 4 data center with RAID2 spinning disk storage, which ...
Centera Content Addressed (CAS) Storage which enhances CAS, includes Event-based Retention and Litigation Hold software to enable healthcare providers more control over information retention periods to provide stricter adherence to HIPAA compliance or governance requirements.


 July 06, 2006
July 06, 2006