CMS has received FDA 510(k) clearance for Monaco, its next-generation IMRT planning platform reportedly featuring innovative biological cost functions, allowing CMS to begin distributing Monaco for clinical use in the U.S.
King Systems Corp. will feature its KING LT-D and KING LTS-D supraglottic airway devices at ASA 2007. The KING LT-D ...
GE Healthcare has released the latest version of Centricity Enterprise, an intelligent and integrated software suite of ...
Radiology departments have many different needs and face a wide variety of challenges that can impact their departments ...
Artromick recently announced the general availability of the NX10 Mobile Computing WorkStation, a point-of-care ...
RIS/PACS solutions provider RADinfo Systems has developed a new software program to allow physicians who are seeking ...
InSite One Inc. will debut its InDex 5 digital medical archive that will contain new features created to improve ...
Despite decades of progress in breast imaging, one challenge continues to test even the most skilled radiologists ...
The CR Pro 2000 features a CR reader that is specifically designed for two markets: the OEM market, for use as a CR ...
The RIS Concepts advanced exam-scheduling wizard for multisite facilities searches across locations to select the first ...
Swissray’s ddRCompact, incorporates the new digital High Definition Silicon Solid State detector with Micro Lens ...
Bayer Radiology’s Barbara Ruhland and Thom Kinst discuss how radiology departments can address the many different ...
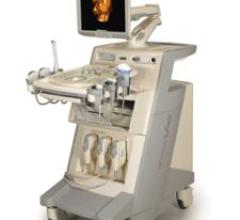
The ACCUVIX V10 is a new product line manufactured by Medison that reportedly incorporates futuristic design with 2D, 3D ...
The LOGIQ I 10-pound system reportedly empowers clinicians with volume imaging and wireless capability for comprehensive ...
Building on the foundation of GE Healthcare’s CT platform, the LightSpeed VCT XT is reportedly capable of capturing ...
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
October 17, 2007 – CMS has received FDA 510(k) clearance for Monaco, its next-generation IMRT planning platform ...
Hemodynamic monitoring technology company Edwards Lifesciences Corp. announced the introduction of its Edwards PediaSat ...
Anesiva Inc. recently announced the FDA has approved Zingo (lidocaine hydrochloride monohydrate) powder intradermal ...
Derma Sciences Inc., a manufacturer and marketer of advanced wound care products, announced it received clearance from ...
The Gammex Neonatal Chest Phantom is the first of a new class of phantoms and objective system performance tools for use ...
Medicsight will be showing its ColonCAD product, integrated with a number of advanced visualization partners, including ...
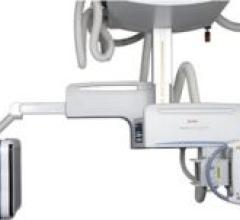
Carestream Health’s newest DR platform, the KODAK DIRECTVIEW DR 9500 has a ceiling-mounted U-arm that contains both a ...
GE Healthcare’s Discovery Dimension helps clinicians advance toward the goal of motion free PET/CT imaging by allowing ...

 October 16, 2007
October 16, 2007