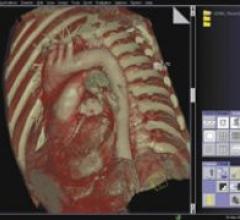
EDDA Technology’s IQQA-Liver Enterprise for contrast CT supports physicians’ real-time evaluation of liver and hepatic lesions on hospital’s existing PACS workstations. By combining advanced image analysis algorithms and visualization tools, the software allows physicians to distill critical diagnostic information from data volume of extraordinary size, according to the company.
April 10, 2008 - Breast Ultrasound Computer Aided Detection (CAD) developer and manufacturer, Medipattern and OEM ...
At SIIM 2008, Cerner will feature its ProVision PACS, which aims to provide a set of user-customizable image-analysis ...
Radiology departments have many different needs and face a wide variety of challenges that can impact their departments ...
Return on investment (ROI) is an important consideration for any capital equipment purchase, and that includes ...
April 10, 2008 - Northwest Hospital and Medical Center in Seattle this week was named among the top 5 percent of ...
M*Modal will showcase its AnyModal CDS Live, which extends the company’s conversational documentation service, AnyModal ...
Despite decades of progress in breast imaging, one challenge continues to test even the most skilled radiologists ...
In the worlds of business, entertainment and politics (including, branding, marketing, newsworthiness and public ...
April 9, 2008 − MediNotes Corp. this week released its latest version of its Web-based integrated electronic medical ...
Integra LifeSciences Holdings Corp. introduced the next generation of MAYFIELD titanium skull pins designed for use with ...
Bayer Radiology’s Barbara Ruhland and Thom Kinst discuss how radiology departments can address the many different ...
Nucletron released three new products designed to improve brachytherapy practice. Designed for use in brachytherapy ...
Heart disease is the leading cause of death of Americans, claiming hundreds of thousands of lives yearly. The ability to ...
The FDA 510(k) cleared the new Fuji DR (FDR) system, Unity SpeedSuite, a single detector designed to initiate image ...
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
The FDA gave Siemens Medical Solutions 510(k) market clearance of four new software applications used with the SOMATOM ...
Carestream Health showcased new capabilities for its Carestream RIS and PACS platforms. New features for Kodak ...
At AACN 2008, Vital Signs Inc. will display its new 500 and 1,000 mL InfusaScan fluid pressure infusor bags, which are designed to help reduce medication errors.
The Health o meter PRO PLUS Electronic Wheelchair Scale has a capacity of 800 pounds and for stable access includes a 10 ...
VersaLights single-use surgical device by Lumitex Medical Devices is reportedly five times brighter, as well as more ...
Elekta’s Synergy, a Modulated and Image Guided Radiation Therapy (IMRT and IGRT) system is designed to provide automated ...
Medrad hopes to thrust new life into molecular imaging with its 510(k) pending FDG-PET power injector system called Intego, designed to promote greater accuracy in dose injection and which reportedly reduces radiation exposure related to FDG-handling by 40 percent.
April 9, 2008 - Engineers at Purdue University are creating a wireless device designed to be injected into tumors to ...

 April 09, 2008
April 09, 2008