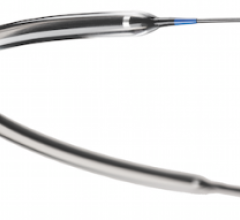
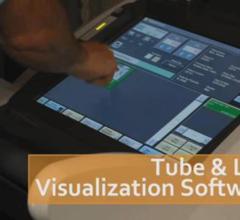
September 24, 2012 — SonoSite Inc., a subsidiary of Fujifilm Corp. and a specialist in bedside and point-of-care ultrasound, has announced a partnership with Soma Access Systems LLC to bring AxoTrack needle visualization technology to market on SonoSite ultrasound systems.
The new U.S. Food and Drug Administration Safety and Innovation Act (FDASIA) imposes many Medical Device Establishment Registration and Listings requirements, effective Oct. 1, 2012. To assist the medical device industry, Registrar Corp has provided the following list of top 10 things that medical device professionals need to know regarding FDASIA:
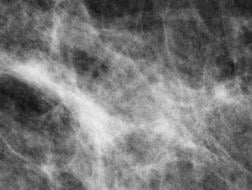
September 24, 2012 — Governor Jerry Brown has signed a bill to improve breast cancer detection in women with dense breast tissue. Senate Bill 1538, authored by State Senator Joe Simitian (D-Palo Alto), will require that following a mammogram, women with dense breast tissue be informed of the following: They have dense breast tissue; that dense breast tissue can make it harder to evaluate the results of a mammogram; that it is associated with an increased risk of breast cancer; that information about breast density is given to discuss with their doctor and that a range of screening options are available.
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
GE Healthcare, a business division of General Electric Company, announced it is joining with RadNet, a network of outpatient diagnostic imaging centers, to pilot its Best Pathways initiative, a breast cancer detection optimization model. GE Healthcare and RadNet will evaluate the clinical breast cancer detection process to quantify inefficient diagnostic patterns and identify optimal paths to lower cost and improve patient outcomes. This program will focus on subpopulations of women in which guidelines are lacking and substantial variability is observed in clinical practice.
Mach 7 Technologies (Mach7), a global provider of enterprise clinical image management solutions, has been named the Global Enterprise Imaging Informatics Entrepreneurial Company of the Year by Frost & Sullivan. The Best Practices accolade comes as a result of Frost & Sullivan’s recent analysis of the enterprise medical image archiving market.
September 20, 2012 — Viztek, a digital imaging solution provider, announced the availability of a wall mounting kit for its small-footprint Opal computed radiography (CR) II X-ray system. The wall-mounted configuration is ideal for a variety of healthcare settings.
While most women understand the importance of health screenings, an estimated 72 million have missed or postponed a ...
September 20, 2012 — Hospitals, medical facilities, doctors and long-term care facilities this fall are stocking shelves and purchasing items from their wish list and budgets before a new tax on most medical products goes into effect in 2013.
The medical community buzzed a couple of weeks ago as word spread of technology guru Vinod Khosla’s prediction that the future of medical practice would be more robot than doctor.
September 19, 2012 — Aycan Medical Systems announced today it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for Aycan mobile, an iPad app for teleradiology.
Fujifilm’s APERTO Lucent is a 0.4T mid-field, open MRI system addressing today’s capability and image quality needs ...
September 19, 2012 — Elekta recently announced the latest production release of its Monaco treatment planning system, Version 3.20, which offers customers improved VMAT (volumetric modulated arc therapy) planning and delivery support, in addition to support for Elekta's Agility 160-leaf multileaf collimator (MLC).
In a recent article, “The AMA Backs Bills to Stop Medicare Imaging Pay Cuts,” the American Medical Association (AMA) once again is fighting to maintain the status quo. They’ve picked a battle that won’t help them win the war.
September 19, 2012 — Heart catheter procedures guided by magnetic resonance imaging (MRI) are as safe as X-ray angiography-guided procedures and take no more time, according to a pilot study conducted at the National Institutes of Health (NIH). The results of the study indicate that real-time MRI-guided catheterization could be a radiation-free alternative to certain X-ray-guided procedures.
SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
September 19, 2012 — Merge Healthcare Inc. announced that its board of directors has retained Allen & Company LLC, a New York-based investment bank, to assist in exploring and evaluating a broad range of strategic alternatives including, but not limited to, a sale of the company or a business combination.
Carestream is changing the DR game and putting you in control of the move to digital.
The Carestream DRX-Revolution is a mobile X-ray system on wheels powered by a wireless DRX detector.
See the versatility of the DRX Evolution room with the wireless DRX detector.
Jeannie Patterson, PSW at Hamilton General Hospital, explains some of the benefits of the Carestream DRX-Revolution ...
Carestream's DRX - transportable / field portable X-ray unit is designed and tested for the rigorous conditions of ...
September 19, 2012 — In response to criticism by physicians about the restrictiveness of conditions put forth by the Center for Medicare and Medicaid Services (CMS) for reimbursement for transcatheter aortic valve replacement (TAVR), the American Society of Echocardiography (ASE) is emphasizing the importance of heart team quality and experience in ensuring positive patient outcomes.
September 18, 2012 — The U.S. Food and Drug Administration (FDA) has approved U-Systems' somo•v automated breast ultrasound (ABUS) system for breast cancer screening as an adjunct to mammography for asymptomatic women with dense breast tissue. With the approval, the somo•v ABUS system becomes the only device approved specifically for screening women with dense breasts.

 September 24, 2012
September 24, 2012