March 21, 2008 - Cordis Corp. has received 510(k) marketing clearance from the FDA for the S.M.A.R.T. Nitinol Stent Transhepatic Biliary System for lengths of 120 mm and 150 mm, the company reported at the 33rd Annual Scientific Meeting of the Society of Interventional Radiology (SIR) meeting.
March 21, 2008 - Drawing on its experience in disease management for Medicaid patients and consumer-based technologies ...
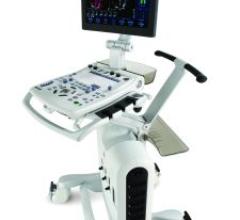
The Vivid S6 signature class cardiovascular ultrasound system reportedly combines strong performance and excellent image ...
Radiology departments have many different needs and face a wide variety of challenges that can impact their departments ...
March 20, 2008 - Drug-resistant tuberculosis is rising to record levels, according to the World Health Organization (WHO ...
March 20, 2008 - New awards geared toward young scientists and medical students who are currently working in or ...
March 20, 2008 - Siemens Medical Solutions has received FDA 510(k) market clearance for the sale and distribution of two ...
Despite decades of progress in breast imaging, one challenge continues to test even the most skilled radiologists ...
The Vivid e adds a fully integrated, three-connector, docking cart, creating a true hybrid system with the power of ...
March 20, 2008 - Harris Corp. was recently awarded a contract by the U.S. Department of Health and Human Services to ...
March 20, 2008 – Siemens Medical Solutions received FDA 510(k) market clearance for the sale and distribution of two ...
Bayer Radiology’s Barbara Ruhland and Thom Kinst discuss how radiology departments can address the many different ...
GE will highlight a new design concept for its Vivid S5 cardiovascular ultrasound system, combining features from its ...
March 20, 2008 - Welch Allyn recently announced at the 2008 HIMSS show that it created a new certification program for ...
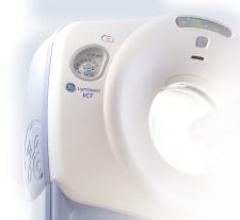
The LightSpeed VCT CT scanner for cardiac imaging reportedly reduces a patient�s radiation exposure by up to 70 ...
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
March 20, 2008 - Intelerad Medical Systems surpassed 500 installations of its IntelePACS, a comprehensive enterprise ...
March 20, 2008 - A new clinical study recently published in the Journal of Emergency Medicine found the Masimo Rainbow ...
March 20, 2008 - Cogent Health Solutions will offer its Equicare CS (cancer survivorship) case management software with ...
March 19, 2008 – American Medical Sales Inc. released MIDAS PACS, including a 100 percent redundant archive and new ...
March 19, 2008 - Xoft Inc. received expanded clearance from the FDA for the Axxent Electronic Brachytherapy System, a ...
American Medical Sales Inc. released MIDAS PACS, including a 100 percent redundant archive and new diagnostic viewing ...
March 19, 2008 - Doctors may one day be able to detect early stages of colon cancer without a biopsy, using a new ...
March 19, 2008 - Baxa Corp. said the latest addition to its STAR (Skills Training, Academics and Resources) Center ...

 March 20, 2008
March 20, 2008