Learn more about myQA, IBA’s unique platform that connects QA applications, people, and know-how through a central database and the Cloud. It offers full support throughout all of your QA, and enables you access to the different software modules and all of your data from one intuitive interface – anywhere and anytime.
December 20, 2016 — The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for Siemens’ Somatom ...
December 20, 2016 — The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance to Statlife’s DenSeeMammo ...
Radiology departments have many different needs and face a wide variety of challenges that can impact their departments ...
December 19, 2016 — Children’s National Health System and Celsion Corp. announced in November the launch of a clinical ...
December 19, 2016 — A recent report from market research firm Signify Research presents five reasons why artificial ...
Client Outlook, a provider of FDA Class II diagnostic and clinical image viewing solutions, announced recently that Chinese company eimageglobal has selected eUnity as its diagnostic enterprise image viewing platform, and both parties have formed a strategic long-term partnership. Eimageglobal’s physician/patient/scheduling platform won the China National Innovation and Entrepreneurial award this year, and the integration of eUnity allows physicians, healthcare providers and patients to have increased and timely access to critical health information.
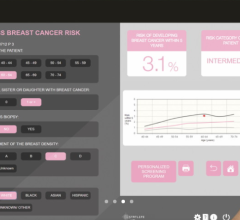
Despite decades of progress in breast imaging, one challenge continues to test even the most skilled radiologists ...
Materialise NV showcased the benefits of 3-D-printed anatomical models for radiologists and their patients during the 2016 Radiological Society of North America (RSNA) Scientific Assembly and Annual Meeting, Nov. 27 – Dec. 2 in Chicago.
Biodex Medical Systems Inc. recently announced the redesign and availability of the new Surgical C-Arm Table 840.
ITN and DAIC Editor Dave Fornell takes a tour of some of the most innovative new technologies being displayed on the ...
Bayer Radiology’s Barbara Ruhland and Thom Kinst discuss how radiology departments can address the many different ...
There were more than 23,600 page views of the video content on the Imaging Technology News (ITN) website in 2016. Here ...
A discussion with Simon Dixon, M.D., MBChB, on the use of fractional flow reserve-computed tomography (FFR-CT) to ...
A post-game roundup by ITN Contributing Editor Greg Freiherr and ITN Editor Dave Fornell on the trends and new tech seen ...
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
At RSNA 2016, the key buzzwords were “deep learning,” “machine learning” and “artificial intelligence.” Vendors and ...
Enterprise imaging is supposed to break down silos so medical images and other patient data can flow freely across campuses, departments, outpatient clinics, and physician offices. Some critics say, however, that in some cases enterprise imaging simply expands radiology's own silo to surround those of other medical specialties.
Patricia Oliveira-Szejnfeld, M.D., and Fernanda Tovar-Moll, M.D., Ph.D., explain what radiologists should be looking for ...
December 14, 2016 — Patients undergoing magnetic resonance (MR) exams will find a relaxing environment with Toshiba ...
Client Outlook announced in November that the U.S. Food and Drug Administration has cleared its eUnity viewing platform for diagnostic reading of mammography images.
Cancer Targeted Technology (CTT), a privately-held Seattle-based biotechnology firm focusing on small molecules that target pivotal enzyme targets on cancer, announced a Phase I clinical trial has commenced in men with high-risk metastatic prostate cancer. In late August, the U.S. Food and Drug Administration cleared CTT’s first Investigational New Drug Application (IND) for a positron emission tomography (PET) imaging agent, CTT1057, labeled with a two-hour half life radiolabel, fluorine-18, and targeting prostate-specific membrane antigen (PSMA).
Clarius Mobile Health announced the U.S. Food and Drug Administration (FDA) has cleared the C3 and L7 Clarius Wireless Ultrasound Scanners, which are compatible with the latest iOS and Android smartphones and tablets.
Here is the list of the top 10 most popular pieces of content on the Imaging Technology News (ITN) magazine website from the month of November based on website analytics:


 December 20, 2016
December 20, 2016