At this year’s RSNA annual meeting, Ziehm Imaging will highlight Ziehm Vision RFD 3-D, a 3-D C-arm with flat-panel technology that provides a 16-cm edge length per scan volume.
ClearDATA and Merge Healthcare Inc. announced that ClearDATA will be the cloud-computing provider for Merge Healthcare's new Merge One solution.
CompView Medical (CVM) debuts the M2u and M4u, its latest design of NuBOOM, an all-in-one equipment manager, visualization and ergonomic boom system.
Radiology departments have many different needs and face a wide variety of challenges that can impact their departments ...
DS debuts at RSNA 2014 the AbbaDox Exchange image and results communication platform, highlighted by the ability to automate distribution of final radiology reports to a referrer’s EHR through Meaningful Use-compliant Direct messaging. The innovative cloud-based technology also automatically transmits a full range of data, including image links and CCD files, to multiple sites across the continuum of care without a complex and costly HL7 feed
The U.S. Food and Drug Administration (FDA) today cleared HeartFlow’s FFR-CT (fractional flow reserve—computed tomography) software, which permits non-invasive evaluation of coronary artery blood flow in patients showing signs of coronary artery disease.
Philips Healthcare has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its IQon Spectral computed tomography (CT) technology for spectral imaging. It adds a new dimension to CT imaging, delivering anatomical information and offering the ability to characterize structures based on their material content within a single scan. The enhanced image quality may help improve confidence in diagnoses and deliver operational efficiency.
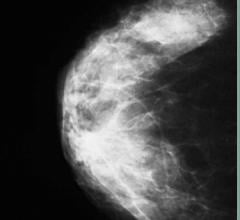
Despite decades of progress in breast imaging, one challenge continues to test even the most skilled radiologists ...
AffordAScan is an online radiology marketplace that makes it easy for patients to compare prices for medical scans at high-quality imaging facilities and request appointments at the same time.
AZE Technology announced the U.S. Food and Drug Administration (FDA) approval of Phoenix (Volume Registration Viewer), a viewer developed with a new concept for efficient and accurate image interpretation.
The Society of Cardiovascular Computed Tomography (SCCT) has published an update of the "SCCT Guidelines for the Interpretation and Reporting of Coronary CT Angiography: A Report of the Society of Cardiovascular Computed Tomography Guidelines Committee" document.
Bayer Radiology’s Barbara Ruhland and Thom Kinst discuss how radiology departments can address the many different ...
Samsung Electronics America Inc. announced availability in the United States of the Samsung RS80A, a high-resolution ultrasound system. Incorporating Samsung’s latest technology, the RS80A offers sophisticated digital image processing and high resolution to support accurate diagnosis.
Fujifilm Medical Systems will introduce its latest digital radiography detectors, FDR D-EVO II at t the Radiological Society of North America (RSNA) in Chicago, Ill. and will be available in the U.S. in early 2015.
A new study by researchers from Icahn School of Medicine at Mount Sinai found that training medical students to use a handheld ultrasound device can enhance the accuracy of their physical diagnosis.
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
medQ, Inc., has released the Patient Balance Estimator, an integrated solution that enables organizations using the Q/ris 3000 to accurately determine a patient’s financial responsibility prior to providing service.
Consensus recommendations are needed in order to guide clinical decisions on the use of image guided radiation therapy in treating pediatric patients, according to a study published in the September-October 2014 issue of Practical Radiation Oncology.
According to a 2014 Benchmark study on Patient Privacy & Data Security, healthcare respondents view the use of cloud services as a serious threat, second only to employee negligence.
Grayhill will display its new optically bonded Instinct touch panel, capable of recognizing up to 10 touch points simultaneously, at the 2014 Radiological Society of North America (RSNA) annual meeting.
EDDA Technology announced that the company has received U.S. Food and Drug Administration (FDA) clearance on IQQA-BodyImaging. The new product will be introduced as the latest addition to the IQQA Platform and Product Suite for imaging-guided cancer treatment at the 100th Scientific Assembly and Annual Meeting of the Radiological Society of North America (RSNA) in Chicago IL Nov. 30-Dec. 5.
Bracco Diagnostics Inc. announced the availability of its Isovue (iopamidol injection) Imaging Bulk Package (IBP), a specific combination multi-patient, multi-dose compliant contrast medium approved by the U.S. Food and Drug Administration (FDA) for point-of-care use in the computed tomography (CT) suite.
The accurate measurement of mammographic density offers the potential to improve breast cancer risk prediction and to tailor screening protocols according to risk, according to a study published in Breast Cancer Research (16:439 doi:10.1186/s13058-014-0439-1). The article, “Digital mammographic density and breast cancer risk: a case-control study of six alternative density assessment methods,” demonstrates a strong connection between breast density and breast cancer risk.
RedRick Technologies, a provider of ergonomic radiology workstations, monitor mounting solutions and reading room environment guidance, has helped the new Penn Musculoskeletal Center implement their newest musculoskeletal radiology reading room, which may also serve as a model for future reading room designs at Penn Medicine.


 November 30, 2014
November 30, 2014