June 9, 2008 – Electronic health records maker MediNotes Corp. said it added 199 new software agreements with new and ...
Candelis received 510(k) marketing clearance for its ImageGrid Mammography Web Viewer and ImageGrid Radiology Web Viewer ...
Acusphere has submitted a New Drug Application (NDA) to the FDA for approval to market Imagify (Perflubutane Polymer Microspheres for Injectable Suspension), an ultrasound imaging agent for the detection of coronary artery disease, which could prove as accurate as nuclear stress testing.
Radiology departments have many different needs and face a wide variety of challenges that can impact their departments ...
The future is looking bright for virtual colonoscopy (CTC) after encouraging news came out that the Centers for Medicare ...
June 9, 2008 - At the request of its membership, the American College of Physicians (ACP) recently conducted an ...
June 9, 2008 - Siemens Medical Solutions gained FDA 510(k) market clearance for the SOMATOM Definition AS, what is said ...
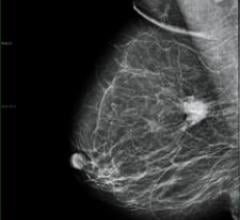
Despite decades of progress in breast imaging, one challenge continues to test even the most skilled radiologists ...
Toshiba Teli America introduced the T24MSA001-MD medical-grade 24-inch LCD color widescreen monitor with full HD (1,080 ...
ProHance is a macrocyclic, nonionic, gadolinium-based magnetic resonance imaging (MRI) contrast agent designed for use in MRI in adults and children over two years of age to visualize lesions with abnormal vascularity in the brain (intracranial lesions), spine and associated tissues. ProHance is also indicated for use in MRI in adults to visualize lesions in the head and neck.
June 9, 2008 - The administration of imprecise dosages, particularly to small children, remains a serious problem faced ...
Bayer Radiology’s Barbara Ruhland and Thom Kinst discuss how radiology departments can address the many different ...
The FDA granted tentative approval for Covidien’s Abbreviated New Drug Application (ANDA) for its Kit for the Preparation of Technetium Tc 99m Sestamibi Injection. Covidien’s tentatively approved product is a generic of Cardiolite, which is a myocardial perfusion imaging agent used for detecting coronary artery disease.
June 9, 2008 - The FDA cleared Mindray’s DC-3 color ultrasound imaging system, equipped with extensive applications in ...
The FDA recently approved Covidien’s contrast delivery system with radiofrequency identification (RFID) technology ...
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
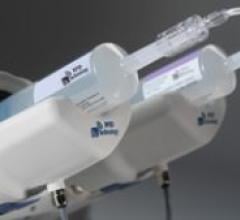
The EmpowerMR injector system has multiple features created to cope with the problem of electrical interference in the ...
With radiologists in short supply, the need for teleradiology services continues to grow and the market has responded in ...
June 9, 2008 – American TeleCare Inc. (ATI) released Quick Notes as a new feature of its inLife/LifeView Telehealth ...
June 9, 2008 - Hologic Inc. said today that it has signed a definitive agreement to acquire Third Wave Technologies Inc ...
GE received FDA clearance for its new Signa MR750 3.0T scanner that delivers up to 60 percent additional anatomical ...
The ACIST CVi Contrast Delivery System provides one injection system for all cardiac and vascular angiography procedures. The variable-rate ACIST CVi system aims to provide ease of operation for clinicians, while obtaining quality images with less contrast used and delivered to the patient.
June 6, 2008 - Misys Healthcare Systems has introduced its new Reseller Partner Program, designed to enable partner ...
June 6, 2008 - InSite One and eHealth Global signed a co-marketing agreement in hopes of offering the medical imaging ...

 June 08, 2008
June 08, 2008