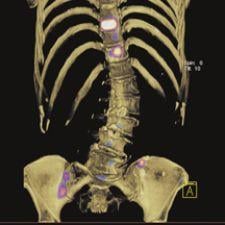
June 13, 2008 - The FDA granted tentative approval for Covidien’s Abbreviated New Drug Application (ANDA) for its Kit ...
Cook Medical's FDA-cleared Evolution Controlled Release Esophageal Stent System is a stent designed to improve the quality of life for patients with esophageal cancer.
June 10, 2008 - World-wide inventors and leaders in radio frequency identification (RFID), bar coding, and other ...
Radiology departments have many different needs and face a wide variety of challenges that can impact their departments ...
Biomarkers are key indicators for pharmaceutical companies in determining which types of drugs to develop for various ...
June 10, 2008 - At the Digestive Disease Week Conference, Cook Medical announced that it has been granted 510(k) clearance from the FDA for use of the Evolution Controlled Release Esophageal Stent System, a stent designed to improve the quality of life for patients with esophageal cancer.
June 9, 2008 – Electronic health records maker MediNotes Corp. said it added 199 new software agreements with new and ...
Despite decades of progress in breast imaging, one challenge continues to test even the most skilled radiologists ...
June 9, 2008 - The FDA cleared Mindray’s DC-3 color ultrasound imaging system, equipped with extensive applications in ...
Toshiba Teli America introduced the T24MSA001-MD medical-grade 24-inch LCD color widescreen monitor with full HD (1,080 ...
ProHance is a macrocyclic, nonionic, gadolinium-based magnetic resonance imaging (MRI) contrast agent designed for use in MRI in adults and children over two years of age to visualize lesions with abnormal vascularity in the brain (intracranial lesions), spine and associated tissues. ProHance is also indicated for use in MRI in adults to visualize lesions in the head and neck.
Bayer Radiology’s Barbara Ruhland and Thom Kinst discuss how radiology departments can address the many different ...
June 9, 2008 - At the request of its membership, the American College of Physicians (ACP) recently conducted an ...
With radiologists in short supply, the need for teleradiology services continues to grow and the market has responded in ...
June 9, 2008 - Hologic Inc. said today that it has signed a definitive agreement to acquire Third Wave Technologies Inc ...
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
The FDA granted tentative approval for Covidien’s Abbreviated New Drug Application (ANDA) for its Kit for the Preparation of Technetium Tc 99m Sestamibi Injection. Covidien’s tentatively approved product is a generic of Cardiolite, which is a myocardial perfusion imaging agent used for detecting coronary artery disease.
June 9, 2008 - The administration of imprecise dosages, particularly to small children, remains a serious problem faced ...
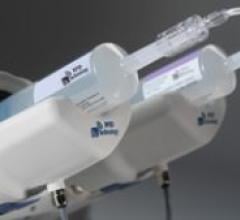
The FDA recently approved Covidien’s contrast delivery system with radiofrequency identification (RFID) technology ...
The EmpowerMR injector system has multiple features created to cope with the problem of electrical interference in the ...
June 9, 2008 - Siemens unveiled the ACUSON SC2000 volume imaging ultrasound system, which acquires nonstitched, real ...
GE received FDA clearance for its new Signa MR750 3.0T scanner that delivers up to 60 percent additional anatomical ...
The ACIST CVi Contrast Delivery System provides one injection system for all cardiac and vascular angiography procedures. The variable-rate ACIST CVi system aims to provide ease of operation for clinicians, while obtaining quality images with less contrast used and delivered to the patient.
June 9, 2008 – American TeleCare Inc. (ATI) released Quick Notes as a new feature of its inLife/LifeView Telehealth ...

 June 09, 2008
June 09, 2008