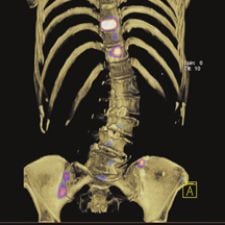
This 99mTc MDP bone SPECT/CT study shows multiple skeletal metastases and was acquired on Siemens' Symbia True Point SPECT/CT.
Biomarkers are key indicators for pharmaceutical companies in determining which types of drugs to develop for various clinical indications. Currently, the role of biomarkers is evolving in the clinical setting, assisting in the design of personalized treatments specific to each patient’s condition and genetic make-up.
Aim of biomarkers
PET, MR, CT and other novel imaging technologies to offer a noninvasive approach to acquiring quantifiable, objective assessments of drug targets, drug performance at the molecular level and pharmacokinetic and pharmacodynamic activity. This capability expedites the clinical trial research process by reducing the number of patients needed in trials and providing earlier assessments of treatment benefit. Because biomarkers are useful in quantifying treatment effect in a clinical trial, they can also be useful in individualizing therapy in clinical practice by monitoring benefit and optimizing the regimen.
“The ultimate aim of a biomarker, strictly speaking, is to aid in drug development,” said a leading expert in the field of biomarker development, Richard Frank, M.D., Ph.D., FFPM, vice president of medical affairs and clinical strategy of medical diagnostics at GE Healthcare. “However, some biomarkers can become diagnostics with potential to identify patients early and then to individualize therapy in a two-step process. First, choose the optimal treatment regimen, and second, monitor therapeutic benefit by testing after treatment starts to ensure the patient is receiving the right drug at the right dose.”
Imaging eyes pharma
Part of GE Healthcare’s strategy to help accelerate the development of new therapeutics includes giving pharmaceutical companies access to novel molecular imaging agents to assess the impact of potential drugs in animal models and, when appropriate, human subjects.
To this end, on May 7 of this year, GE Healthcare signed a nonexclusive agreement with Merck and Co. to share technology on imaging of the lungs that may help to advance respiratory treatment development. In the deal, Merck will have access to Spin Signal Technology (SST) utilizing hyperpolarized Xenon 129 gas, a molecular imaging agent that is under investigation by GE Healthcare to provide high-speed, quantitative imaging of the lung using magnetic resonance imaging (MRI).
This technology is being evaluated to determine if it can provide more sensitive information than currently available tests on how diseased lungs function. By using xenon, a gas that can be modified with the SST to be detectable with MRI, the MRI generates images that may provide clinical value by allowing regional imaging of disease. This information could enhance that provided by FEV1, the volume exhaled during the first second of a forced expiratory maneuver.
“Imaging the lungs has traditionally been challenging for pharmaceutical companies needing to assess the impact of developmental therapies on lung function. This agreement enables the companies involved in this collaboration to assess in real time the effect of potential therapeutics in animal models,” said Jonathan Allis, head of the global imaging network at GE Healthcare.
“With cutting-edge molecular diagnostic capabilities across all modalities, GE Healthcare is well positioned to work with pharmaceutical companies to tailor therapeutic assessment programs to further their drug development,” said Kim Gallagher, head of external scientific affairs in the Medical Diagnostics Division of GE Healthcare. “By joining this development collaboration, Merck will have full access to the SST and hyperpolarized Xenon 129 trial data to monitor its potential and plan for its possible incorporation in therapy trials. We look forward to initiating further agreements with key pharmaceutical companies in the coming year in other disease areas.”
Biomarkers target the next step
Personalized medicine, as it’s referred to by the Society of Nuclear Medicine, is tailored medical treatment based on a person’s unique molecular profile for the detection, treatment or prevention of disease. While many people equate personalized medicine with genomics, Dr. Frank pointed out, “Genomics can only assess risk or likelihood of disease, likelihood of response. Biomarkers carry this to the next step by actually identifying early stages of pathophysiology, such as CT angiography finding blockages in coronary arteries before a heart attack, or PIB-PET finding Abeta plaque in the brain during the five to eight years of Alzheimer’s disease before symptoms even appear.” In essence, biomarkers aid in early detection at which point healthcare providers may tailor treatment at this early stage of disease.
Gary Small, M.D., professor of psychiatry and aging at the University of California at Los Angeles Femel Institute, who researched FDG-PET in differentiating cognitive impairment, said, “The idea [behind his research into the early detection of Alzheimer’s disease] is to try to protect the healthy brain rather than trying to repair it once the damage has already been done.”
Although personalized medicine is practiced today, many improvements can still be made in the area of cost and time efficiency and patient safety. “In fact, we already have this [personalized medicine] today, but it happens only by a long, drawn-out process of hit-and-miss,” Dr. Frank explained, “For example, anti-hypertensives are generally prescribed in low doses and then the dose is increased, or the drug is changed, when the blood pressure does not come down. Then a second and third drug is added when this fails. When we know more about genetic risks, and when we know more about subsets of disease — stratifying patient populations — we’ll do a better job of starting out on the right medicine, saving time and money and adverse events.” <


 February 13, 2026
February 13, 2026 









