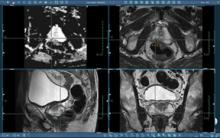
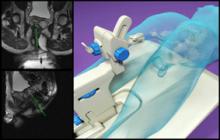
November 5, 2010 – The U.S. Food and Drug Administration (FDA) has given 510(k) clearance for an advanced dose calculation algorithm. The Acuros XB, by Varian Medical Systems, can complete a RapidArc treatment plan two to three times faster than conventional algorithms.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now