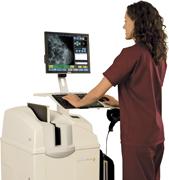
November 4, 2010 – The U.S. Food and Drug Administration (FDA) has cleared a high-resolution computed radiography (CR) system for mammography. The Directview CR system, by Carestream, is intended for use in the same clinical applications as traditional screen-film based mammography systems.
A mammography feature enables images to be captured digitally while utilizing a clinic’s existing X-ray unit.
"Annual mammograms for women are important for good health and our CR mammography system will provide healthcare organizations with yet another option for providing high-quality screening for breast cancer," said Dina Vazzana, general manager, women’s healthcare, Carestream Health. "This high-performance, cost-effective system will enable medical facilities to benefit from advanced digital medical imaging technology in a way that complements their patient-focused operations."
Carestream Health’s CR mammography feature is available in Europe, Greater Asia, Japan, Latin America and Canada.
For more information: www.carestreamhealth.com


 February 17, 2026
February 17, 2026 









