Artera is leading the way in developing multimodal AI biomarkers to stratify cancer risk. (Image: Getty Images)
Genomics has guided personalized cancer treatments for the past two decades. Now, AI biomarkers are expanding the field, helping radiologists and radiation oncologists stratify risk and potentially plan more precise treatments.
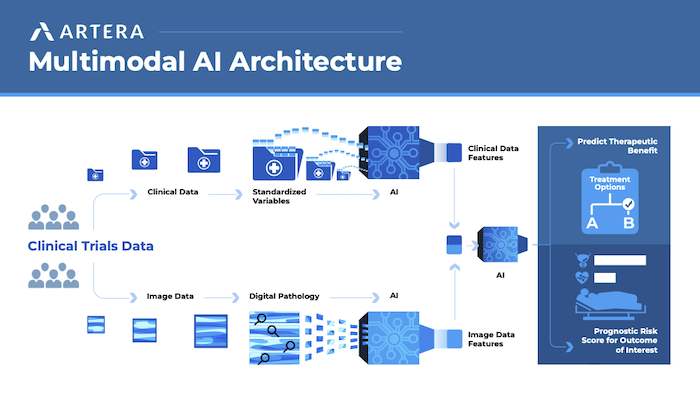
Unlike traditional biomarkers, “AI analyzes images of a patient’s biopsy to predict how aggressive a patient’s cancer might be, or whether they may respond better to treatment A or treatment B,” says Erin Stewart, vice president of clinical development for Artera, a developer of multimodal AI (MMAI)-based prognostic and predictive cancer tests.
Four abstracts presented at the 67th annual American Society for Radiation Oncology (ASTRO) meeting in September/October in San Franscisco, including the first validation of the Artera Prostate Test in an Asian patient cohort1, offered new insights into the growing role of AI biomarkers.
Accuracy in Underrepresented Populations
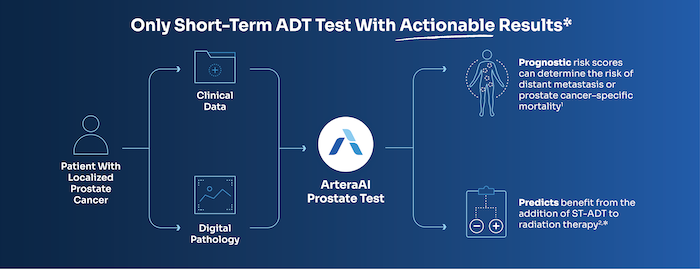
The validation study, conducted in Asian prostate cancer patients from Singapore, supported the performance of a biopsy MMAI model across different racial subgroups. The study evaluated the Artera Prostate Test — the first AI risk stratification tool recommended for patients with localized prostate cancer in National Comprehensive Cancer Network (NCCN) Guidelines.2
“As a physician scientist, it’s natural to be cynical when you see a tool like that because the results are too good to be true,” says radiation oncologist Melvin L.K. Chua, FRCR, PhD, FASCO, from the National Cancer Centre Singapore, a lead author of the study. “We tested the model in an independent dataset to see if it actually pans out. Just by simply doing the test in a remote site where there were no prior collaborations and then seeing the very clean result, that’s important to the scientist and to the consumer.”
The findings build on data from the 2024 American Society of Clinical Oncology (ASCO) meeting showing strong predictive accuracy in African American men, another historically underrepresented group in biomarker research.
“We know from history that biomarkers can have biases, so it’s important to study these biomarkers in different populations, regions, and racial groups,” Stewart says.
Expanding MMAI to Other Cancers
A second abstract validated the use of an Artera-developed MMAI biomarker in patients with head and neck cancer. The biomarker was prognostic for survival and disease progression, regardless of stage or human papillomavirus status, in patients treated with either definitive radiation or surgery.3
Notably, the head and neck biomarker was developed in just five months, Dr. Chua says. “With the prostate work, it took time to learn the data and best practices. Once Artera had that right, the framework works across other cancer types, so you can move a lot faster.”
Two other abstracts evaluated the correlation between genomic classifiers and digital pathology-based MMAI in localized prostate cancer.4,5 “While there’s some comparability in performance, there are still features the MMAI picks up that aren’t reflected in molecular biology, and vise versa,” Dr. Chua says. “We’re just at the tip of the iceberg, and these studies make the science more robust and enhance our understanding of how these biomarkers work.”
Practical Implications of MMAI
While AI biomarkers are still in their early stages, Dr. Chua sees clear potential for clinical impact, especially in prostate cancer, where treatment intensity varies widely.
“If a patient with intermediate-risk prostate cancer has a low-risk result, providers could then discuss whether there’s a need for hormonal therapy,” Dr. Chua says. “That might save the patient a year of low-testosterone symptoms.”
The Road Ahead
Artera plans to expand its AI biomarkers across multiple cancer types, starting with breast cancer. The company has secured UKCA certification for two additional tests — a breast test and a prostate biopsy assay — with others in active development.6
“We have research collaborations on every single continent, excluding Antarctica,” Stewart says. “There’s real excitement about how this technology can help increase and improve access to precision oncology tools.”
Dr. Chua is optimistic about the future of precision oncology. “[It] is not a figment of imagination,” he says. “It’s going to happen in the clinic and be pervasive. We want this to reach everywhere around the world.”
References
- Ong EHW, Hong BH, Joun S, et al. Validation of a prognostic multimodal artificial intelligence (MMAI) model in Asian prostate cancer (PCa) patients from Singapore. Int J Radiat Oncol Biol Phys. 2025;123(1)(suppl):S232. doi:10.1016/j.ijrobp.2025.07.1355
- Artera. For Clinicians. Artera website. https://artera.ai/for-clinicians#nccn-table. Accessed Nov. 21, 2025.
- Chinn SB, Ma X, Tierney M, et al. Development and validation of a digital pathology–based, multimodal artificial intelligence (MMAI) biomarker in patients with head and neck cancer. Int J Radiat Oncol Biol Phys. 2025;123(1)(suppl):S72-S73. doi:10.1016/j.ijrobp.2025.06.1087
- Trivedi P, Awasthi S, Putney R, et al. Genomic classifier and multimodal AI biomarker in localized prostate cancer: two sides of the same coin. Int J Radiat Oncol Biol Phys. 2025;123(1)(suppl):S167–S168. doi:10.1016/j.ijrobp.2025.06.1271
- Olabumuyi AA, Jordan O, Chang JH, et al. Evaluating the correlation between genomic classifier and digital pathology–based multi-modal AI biomarkers in localized prostate cancer. Int J Radiat Oncol Biol Phys. 2025;123(1)(suppl):e504. doi:10.1016/j.ijrobp.2025.06.2653
- HLTH. FDA approves Artera Prostate as first AI tool for cancer prognosis. HLTH website. https://hlth.com/insights/news/fda-approves-arteraai-prostate-as-first-…. Accessed Nov. 21, 2025.


 March 03, 2026
March 03, 2026 









