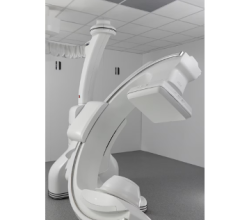
June 19, 2024 —Royal Philips, a global leader in health technology, today announced the launch of Philips Image Guided Therapy Mobile C-arm System 9000 – Zenition 90 Motorized, designed to help clinicians deliver high-quality care to more patients. Philips is partnering with its customers to improve productivity. The new mobile C-arm with expanded capabilities is designed to meet complex vascular needs, but also a range of clinical procedures such as cardiac interventions, pain management and urology. Philips will be showcasing its newly introduced mobile C-arm at the 2024 Society for Vascular Surgery Annual Meeting, June 19-22, in Chicago.
Increased control and efficiency with automated workflows
The Philips Zenition Image-Guided Therapy Mobile C-arm Systems bring together innovations in image capture and processing, ease-of-use, and versatility, many of which were pioneered on Philips’ highly successful image guided therapy platform Azurion. Motorized and impressively fast, the Zenition 90 Motorized is an intuitive C-arm that allowsclinicians to control it from the table-side with user-friendly controls and time-saving features – empowering the clinician with greater flexibility and independence. It delivers state-of-the-art image quality for the most challenging procedures and is designed to meet complex procedural needs. The system allows greater clinical efficiency thanks to its automated workflows, the image controls via the Touch Screen Module and the advanced software solutions.
“During complex procedures, it’s vital to be able to rely on surgical imaging systems. As clinicians navigate their way through challenging anatomy, the priority is to quickly visualize small anatomical details while limiting X-ray dose,” said Mark Stoffels, Business Leader Philips Image Guided Therapy Systems. “The new Zenition 90 Motorized empowers medical teams to confidently perform a wide range of interventions while achieving the best possible outcome for their patients.”
In independent hands-on usability studies of clinicians from the US and EU with the Zenition 90 Motorized in simulated environments; 100% of users said that with the Table Side Operator, they have complete control over C-arm movements [2] and 97% report that workflow features such as Automatic Vascular Outlining will help save time during procedures [3].
As part of its commitment to sustainability and providing customers with responsible choices, Philips leveraged its EcoDesign process for the Zenition 90 Motorized to improve product life by 25% and power efficiency by 13% [4].
Philips latest image guided therapy mobile C-arm system is also available in a non-motorized configuration.
For more information: www.philips.com
Sources
[1]Also available in 15 kW
[2] Results obtained during claims substantiation study performed in June 2022 and May 2023 by Use-Lab GmbH, an independent company. Response is based on 25 physicians from the EU and the US, who answered a questionnaire after a usability study with additional hands-on time with the system.
[3] Results obtained during claims substantiation study performed in June 2022 and May 2023 by Use-Lab GmbH, an independent company. Response is based on 49 clinicians from the EU and the US, who answered a questionnaire after a usability study with additional hands-on time with the system.
[4] Compared to its predecessor, Zenition 70
- Zenition 90 Motorized and Zenition 90 are available for sales in a limited number of countries.
- Some features are optional for Zenition 90 Motorized and Zenition 90.
- Actual product representation may vary.


 February 24, 2026
February 24, 2026