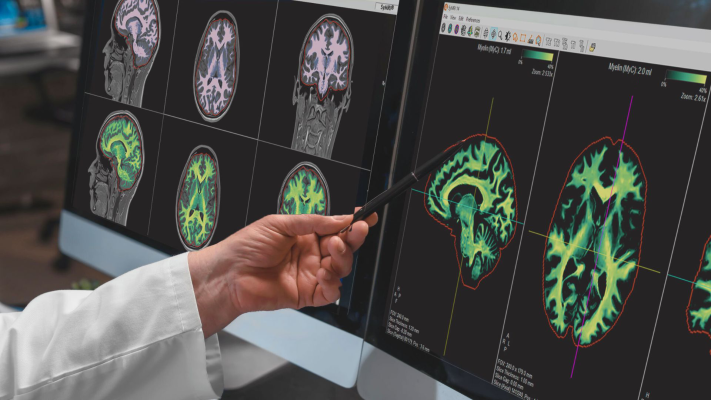
March 27, 2024 — SyntheticMR announced that its next-generation imaging solution, SyMRI 3D, has received FDA 510(k) clearance for clinical use in the United States. This milestone marks a significant advancement in quantitative MRI technology, offering unprecedented resolution and accuracy in brain imaging.
"We are thrilled to receive 510(k) clearance for SyMRI 3D," says Ulrik Harrysson, CEO at SyntheticMR AB. "SyMRI 3D represents the next generation of quantitative MRI, revolutionizing the landscape of medical diagnostics and offering new possibilities for diagnosis and treatment."
SyMRI 3D enables precise volumetric estimations of brain regions, a technique commonly referred to as parcellation, which empowers clinicians to gain deeper insights into brain structure and function. Furthermore, the resolution provided by SyMRI 3D facilitates comprehensive lesion analysis, ensuring a more accurate and in-depth assessment of medical conditions.
"Receiving 510(k) clearance for SyMRI 3D allows us to empower physicians to make more precise and informed decisions in diagnosis and treatment planning through quantitative imaging," says Jared Dixon, President of SyntheticMR U.S Inc.
With this clearance, SyntheticMR reaffirms its commitment to advancing medical imaging technology and providing clinicians with innovative tools to enhance patient care. SyMRI 3D opens up new possibilities for precise diagnosis, treatment planning, and monitoring, ultimately improving patient outcomes.
This disclosure contains information that SyntheticMR AB is obliged to make public pursuant to the EU Market Abuse Regulation (EU nr 596/2014). The information was submitted for publication, through the agency of the contact person, on 27-03-2024 11:01 CET.
For more information: www.syntheticmr.com
Related content:
SyMRI 3D by SyntheticMR Set for Early 2024 Commercial Launch Post Obtaining CE-Marking
SyntheticMR Has Submitted Their Next Generation Solution, SyMRI 3D for 510(k) Clearance


 February 13, 2026
February 13, 2026 









