Getty Images
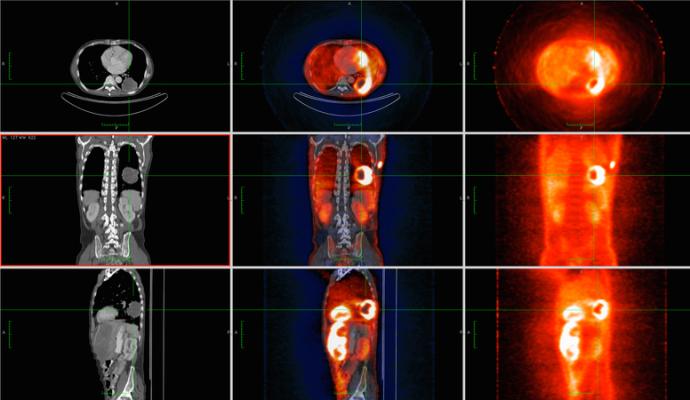
December 6, 2023 — Philochem AG, a wholly owned subsidiary of Philogen S.p.A., and Blue Earth Diagnostics, a Bracco company and recognized leader in the development and commercialization of innovative PET radiopharmaceuticals, today provided additional information regarding a March 2022 licensing and collaboration agreement between Philochem and Blue Earth Diagnostics. The collaboration encompasses the development of OncoFAP, a proprietary, small organic ligand with ultra-high affinity to Fibroblast Activation Protein (FAP), for applications in the diagnostic imaging of solid tumors. Under the agreement, the Philogen Group is conducting a Phase 1 clinical study to evaluate the safety and dosimetry of 68Ga-OncoFAP (68Ga-OncoFAP-DOTAGA) in patients with solid tumors. The agreement provides Blue Earth Diagnostics rights to develop and commercialize 68Ga-OncoFAP worldwide. FAP is a tumor-associated antigen which is expressed in more than 90% of epithelial cancers (e.g., malignant breast, colorectal, ovarian, lung, skin, prostate, and pancreatic cancers, as well as in some soft tissue and bone sarcomas).
The Philogen Group has a Phase I clinical trial (called FAPrimo) underway in Europe to evaluate the safety and dosimetry of 68Ga-OncoFAP in patients with solid tumors. The study is planned to enroll up to 20 patients who will undergo PET/CT imaging with 68Ga-OncoFAP. The first three enrolled patients in the trial were imaged in November 2023 at the National Cancer Institute of Milan (INT). To date, 68Ga-OncoFAP-DOTAGA has been used for PET imaging applications in approximately 100 patients with various solid tumors through Philochem’s collaboration with the University of Münster, Germany.
Prof. Dr. Dario Neri, Member of the Board of Philochem and CEO of the Philogen Group, commented: “We are excited about the promising tumor-targeting performance of 68Ga-OncoFAP in preclinical models and human patients to date. Philochem discovered and published the first paper on this important tumor targeting ligand in 2021. We believe OncoFAP technology has potential to pave the way for a new approach to tumor diagnosis, and significantly improve diagnosis and informed management for patients who have solid tumor cancers.”
“We are delighted to announce this key milestone of the first three patients being dosed in the Phase 1 trial for 68Ga-OncoFAP PET in the detection and localization of advanced solid tumors,” commented David Gauden, D.Phil., Chief Executive Officer of Blue Earth Diagnostics. “68Ga-OncoFAP is an innovative and promising addition to Blue Earth Diagnostics’ robust portfolio of precision PET radiopharmaceuticals. We are grateful for the support of our parent company, Bracco Imaging, S.p.A., in helping to advance innovation to shape the future of precision medicine and improve patients’ lives worldwide with imaging technology such as OncoFAP. Based on preclinical studies and early clinical experience, 68Ga-OncoFAP has demonstrated favorable radiochemical properties, rapid clearance from organs and soft tissues and avid tumor uptake. We look forward to continuing collaboration with Philochem throughout this Phase 1 trial and applying our deep expertise in radiopharmaceutical development and commercialization as we advance further, with the goal to help patients and address unmet needs in cancer.”
About Fibroblast Activation Protein (FAP) and FAP-targeted Technology
Fibroblast Activation Protein, or FAP, is a transmembrane protein that is overexpressed in cancer-associated-fibroblasts (CAFs) in the tumor microenvironment of most tumor types, but expressed at low levels in normal adult tissues. It is involved in a variety of tumor-promoting activities and is considered a promising target with diagnostic and theranostic potential in a variety of diseases. FAP is being investigated for use in diagnostic imaging and therapy in multiple tumor types, such as colon, breast, lung, pancreatic and esophageal cancers, among others. CAFs are also highly expressed in tissue remodeling, and FAP imaging may have applications in benign fibrotic conditions such as wound healing, as well as in chronic inflammation, cirrhosis, and cardiovascular and rheumatoid diseases.
Radiolabeled FAP ligands have demonstrated high binding affinity, rapid binding to FAP-expressing tumors and impressive lesion to background ratios in patients with a broad range of cancer types. They can be labeled with isotopes such as 68Ga or 18F for diagnostic PET imaging, or with therapeutic radioisotopes such as 177Lu or 225Ac for therapy.
For more information: www.blueearthdiagnostics.com


 February 13, 2026
February 13, 2026 









