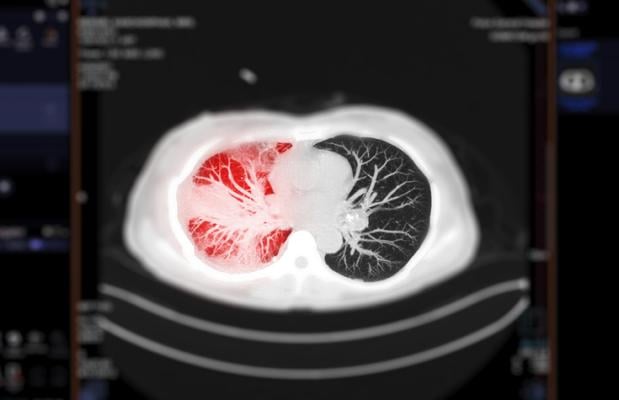
February 10, 2022 — The Centers for Medicare & Medicaid Services (CMS) just announced a national coverage determination (NCD) that expands coverage for lung cancer screening with low dose computed tomography (LDCT) to improve health outcomes for people with lung cancer. Lung cancer is one of the most common cancers and the leading cause of cancer-related death in both men and women in the United States. This screening is aimed at early detection of non-small cell lung cancer.
This final decision expands eligibility for people with Medicare to get lung cancer screening with LCDT by lowering the starting age for screening from 55 to 50 years and reducing the tobacco smoking history from at least 30 packs per year to at least 20 packs per year. The only recommended screening test for lung cancer is LDCT, a unique computed tomography (CT) scan technique that combines special x-ray equipment with sophisticated computers to produce multiple, cross-sectional images of the inside of the body.
“Expanding coverage broadens access for lung cancer screening to at-risk populations,” said CMS Chief Medical Officer and Director of the Center for Clinical Standards and Quality, Lee Fleisher, M.D. “Today’s decision not only expands access to quality care but is also critical to improving health outcomes for people by helping to detect lung cancer earlier.”
The final decision also simplifies requirements for the counseling and shared decision-making visit and, based on public comments received on the proposed NCD and additional review, removes the requirement for the reading radiologist to document participation in continuing medical education, thereby reducing administrative burden on providers. CMS also added a requirement back to the NCD criteria for radiology imaging facilities to use a standardized lung nodule identification, classification, and reporting system.
To read the final decision, visit the CMS website at: https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304
Related content on cancer screening:
Radiation Oncologists Applaud Biden-Harris Administration's Renewed Commitment to Cancer Moonshot
Racial/Ethnic Disparities Persist in Lung Cancer Screening Eligibility
Primary Lung Cancers Detected by LDCT are at Lower Risk of Brain Metastases
Physician and Patient Groups Call On CMS to Update Medicare Lung Cancer Screening Coverage
USPSTF Expands Lung Cancer Screening Eligibility Thresholds
Low-dose CT for Lung Cancer Screening: Benefit Outweighs Potential Harm
Physician and Patient Groups Call On CMS to Update Medicare Lung Cancer Screening Coverage


 March 06, 2026
March 06, 2026 









