October 20, 2020 — Ezra, a NY-based startup transforming early cancer screening using magnetic resonance imaging (MRI), announced that it has received FDA 510(k) premarket authorization for its Artificial Intelligence, designed to decrease the cost of MRI-based cancer screening, assisting radiologists in their analysis of prostate MRI scans. It is the first prostate AI to be cleared by the FDA.
Due to the increased efficiency that Prostate AI will generate for partner radiologists, Ezra is decreasing the price of the Ezra Prostate Scan by 15%, to $575.
"Over the past two years, our team has worked tirelessly on building Ezra's Prostate AI and I'm thrilled to bring it to our imaging partners across the US," said Emi Gal, CEO and co-founder of Ezra. "We will continue to work towards making the interpretation of prostate MRI scans faster and more affordable, in order to support the millions of men who are at risk of prostate cancer."
The Ezra AI achieves three important goals:
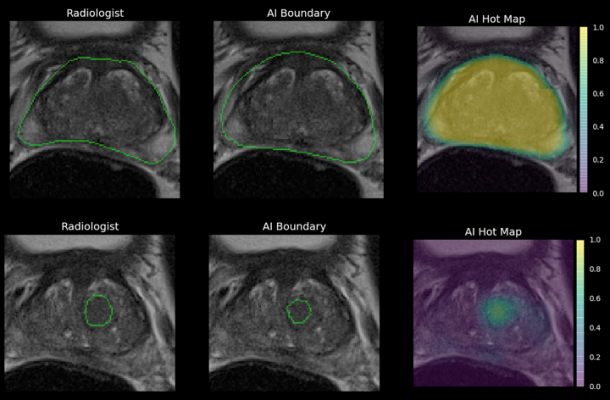
- Accurate prostate volume measurements. Accurate prostate volume is critical to diagnosis because the size of the prostate is often an indication of potential disease.
- Semi-automatic lesion measurements. Under current processes, if a lesion is identified in the prostate, a radiologist has to manually measure the size of the lesion, and grade it using the PI-RADS methodology. The Ezra Prostate AI automatically measures the size of a lesion saving time and minimizing patient worry.
- Automatic prostate and lesion segmentation and 3D volume representation. Should a lesion require a biopsy, the Ezra Prostate AI automatically segments the prostate gland and the lesion for the radiologist for greater accuracy and precision.
To integrate Prostate AI into radiology workflows, Ezra has also obtained FDA clearance for Plexo, a cloud-based PACS (Picture Archiving and Communications System) that works directly in the browser. This means that radiologists can now log onto the Ezra platform and use the AI without the need to install any software.
"Ezra is at the forefront of MRI-based cancer screening, and the company's 510k FDA clearance for its Prostate interpretation AI is further validation of its innovation capabilities," said Lawrence Tanenbaum, M.D., FACR, Vice president and CTO, Director of MRI, CT and Advanced Imaging at RadNet.
Ezra launched its MRI-based prostate cancer screening program in January 2019, and rolled out its full-body MRI scan in May 2019. Ezra partners with existing outpatient imaging facilities, and all Ezra scans are analyzed by board-certified radiologists. The company's cancer screening programs are live in New York City, San Francisco, and Los Angeles through its partnership with RadNet, Inc., the nation's leader in outpatient imaging. In 2019, Ezra helped 4% of its members - all of whom were asymptomatic - detect cancer.
In June, the company announced Ezra COVID 360, a low-dose CT scan of the lungs to look for damage caused by COVID-19, paired with an antibody blood test to check for igG and igM antibodies. The new plan is designed for individuals who want to understand the potential long-term impact of COVID-19 on their lung health and check their antibody status. Ezra COVID 360 is currently available for $390 in New York and California.
For more information: www.ezra.com


 February 06, 2026
February 06, 2026 









