Image courtesy of Clinica Universidad de Navarra, Spain
August 19, 2020 — The Food and Drug Administration (FDA) has cleared two additional Siemens Healthineers artificial intelligence-based software assistants in the AI-Rad Companion family. Both new software assistants free radiologists from routine activities during magnetic resonance imaging (MRI) examinations. The AI-Rad Companion Brain MR for Morphometry Analysis automatically segments the brain in MRI images, measures brain volume, and marks volume deviations. The AI-Rad Companion Prostate MR for Biopsy Support automatically segments the prostate on MRI images and enables radiologists to mark lesions, facilitating targeted prostate biopsies.
“These new AI-Rad Companion applications for MR exams in the brain and prostate regions will help physicians manage their workloads and achieve a patient-focused decision-making process to increase efficiency and improve the quality of care,” said Peter Shen, Vice President of Innovation and Digital Business at Siemens Healthineers North America.
The AI-Rad Companion Brain MR for Morphometry Analysis supports brain volumetry, which involves measuring the volume of gray matter (nerve cells), white matter (nerve cell connections), and cerebrospinal fluid in various segments of the brain and comparing the results to normal volumes. In typical clinical presentation and when combined with independent confirmation, reduced volume may indicate Parkinson’s disease, Alzheimer’s disease, or other forms of dementia.¹ Previously, segmentation and comparison to the norm were performed manually or semi-automatically.
Based on AI algorithms, the AI-Rad Companion Brain MR for Morphometry Analysis can automatically identify roughly 30 brain segments on MRI images, measure their volumes, and compare the results to data in a normative reference database for brain morphometry from the Alzheimer’s Disease Neuroimaging Initiative (ADNI).² The AI-Rad Companion Brain MR for Morphometry Analysis feeds the results into a report where deviations from the norm are marked automatically.
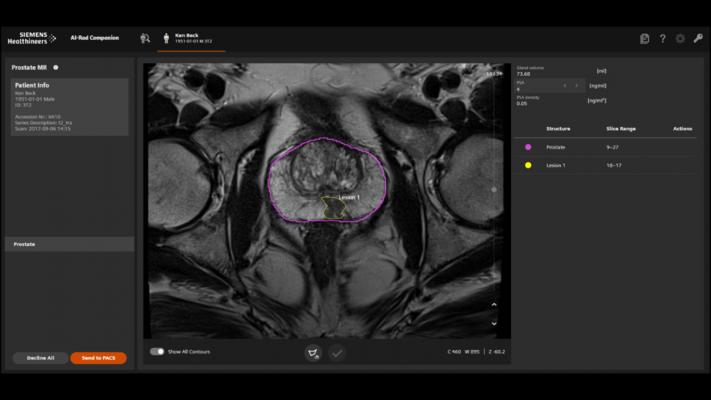
The AI-Rad Companion Prostate MR for Biopsy Support automatically segments the prostate and marks the organ’s outer contour in seconds rather than minutes, as is the case with manual segmentation. The radiologist then simply marks the suspect areas and sends the annotated MRI images to the urologist for fusion with ultrasound images during the biopsy. These targeted, MRI-supported biopsies can help the urologist detect significant prostate carcinomas and improve patient care.
Both new AI-Rad Companion software assistants can be used on MRI scanners from Siemens Healthineers and outside manufacturers, and are available on the teamplay cloud-based digital health platform³ from Siemens Healthineers. These two new assistants join the growing AI-Rad Companion family, which is seamlessly integrated into the existing clinical workflow and is DICOM (Digital Imaging and Communications in Medicine) compliant.
¹ Wolk, D.A., & Dickerson, B.C. (2018). “Clinical features and diagnosis of Alzheimer disease,” UpToDate.com https://www.uptodate.com/contents/clinical-features-and-diagnosis-of-alzheimer-disease?search=Clinical%20features%20and%20diagnosis%20of%20Alzheimer%20disease&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1,
³ teamplay is not yet commercially available in all countries. For regulatory reasons, its future availability cannot be guaranteed. Please contact your local Siemens Healthineers organization for more details.
For more information: www.siemens-healthineers.us/ai-rad


 February 05, 2026
February 05, 2026 









