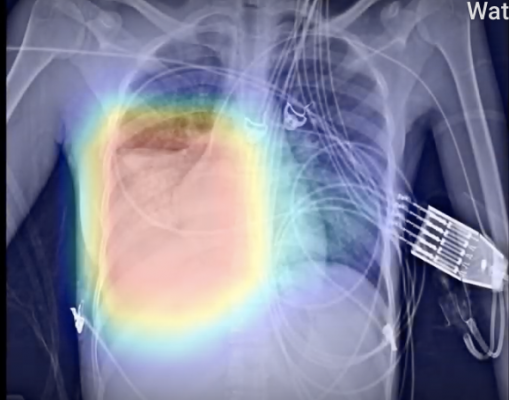
March 23, 2020 — behold.ai announced that its artificial intelligence-based red dot algorithm quickly identifies chest X-rays from COVID-19 patients as ‘abnormal’. This instant triage could potentially speed up diagnosis of COVID-19 individuals and ensure resources are allocated properly.
“The majority of deaths from COVID-19 are owing to pneumonia in the lungs of vulnerable patients. Pneumonia is a potentially life-threatening condition caused by a number of pathogens including, directly or indirectly, COVID-19 infection. Our algorithm can detect abnormal chest X-rays including pneumonia almost instantly. Out of 28 X-rays reviewed from patients with COVID-19, we correctly identified 85% of them as ‘abnormal’ using red dot,” said Tom Naunton Morgan, M.D., MB, FRCS, FRCR, Chief Medical Officer, behold.ai. “As we evaluate further positive cases from across the world, including here in the UK, our results will be further validated. This will increase the utility of our ‘instant triage’ and potentially help reduce the burden on healthcare systems as more and more cases of pneumonia present and require rapid diagnosis.”
The data follows recent news that behold.ai’s algorithm has been cleared by the US Food and Drug Administration (FDA). Commercial rollout in the U.S. is planned for later this year.
“Our technology can make a big difference to patient safety, and the delivery of care and cost-savings to health services. It is available here and now to help manage the increased burden that will fall on health systems like the NHS in the coming weeks,” said Simon Rasalingham, Chairman and Chief Executive, behold.ai.
For more information: www.behold.ai


 February 13, 2026
February 13, 2026 









