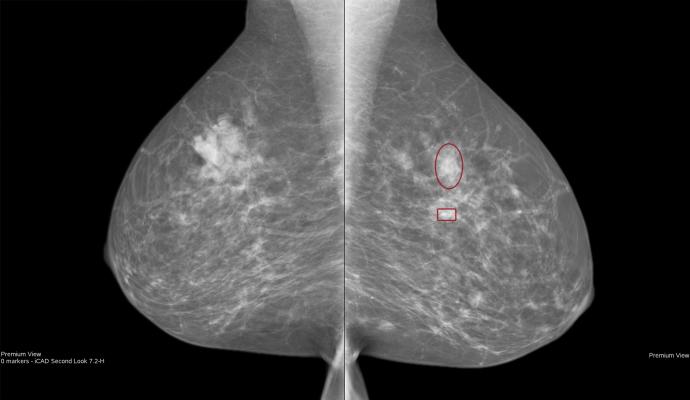
June 13, 2019 — iCAD Inc. announced the launch of ProFound AI for 2D Mammography in Europe. This software is the latest addition to iCAD’s deep-learning, artificial intelligence (AI) platform and follows the launch of ProFound AI for Digital Breast Tomosynthesis (DBT), which was CE Marked in March 2018 and U.S. Food and Drug Administration (FDA)-cleared in December 2018. ProFound AI for 2D Mammography and ProFound AI for DBT will both be featured in the iCAD exhibition booth at the Société Française d'Imagerie de la FEMme (SIFEM) medical conference June 13-15, 2019, in Lille, France.
ProFound AI is available for both mammography and tomosynthesis modalities; it is a high-performance, deep-learning, breast cancer detection and workflow solution that offers benefits to radiologists and patients alike. ProFound AI for DBT is clinically proven to improve cancer detection rates by 8 percent, reduce unnecessary patient recall rates by 7.2 percent, and slash reading time for radiologists by 52.7 percent and up to 57.4 percent for dense breast 3-D image analysis.1
“The arrival of the ProFound AI platform has completely changed the way we read tomosynthesis cases. It is a very powerful and extremely useful tool that improves our diagnostic confidence; especially for subtle lesions and dense breasts,” according to Patrick Toubiana, M.D., radiologist, president and cofounder at the C.S.E. Centre Imagerie Médicale Numérique in Paris. “In addition, ProFound AI allows us to optimize our workflow for better reading comfort and a reduction of the interpretation time of the tomosynthesis slices. Today, ProFound AI is a real asset in our daily practice that we could not do without.”
ProFound AI for 2D Mammography and ProFound AI for DBT were designed to rapidly and accurately analyze each image. They provide radiologists with key information, such as certainty of finding lesion and case scores, which assists in clinical decision-making and prioritizing caseloads. Featuring the latest in deep learning AI, the platform also allows for continuously improved performance via ongoing updates.
ProFound AI for 2D Mammography is pending CE Mark in the EU.
For more information: www.icadmed.com
Related Content
FDA Clears iCAD's ProFound AI for Digital Breast Tomosynthesis
VIDEO: How iCad Uses AI to Speed Breast Tomosynthesis
Reference
Hoffmeister J. (2018). Artificial Intelligence for Digital Breast Tomosynthesis – Reader Study Results. [White paper]. Accessed June 4, 2019 via https://www.icadmed.com/assets/dmm253-reader-studies-results-rev-a.pdf


 March 06, 2026
March 06, 2026 









