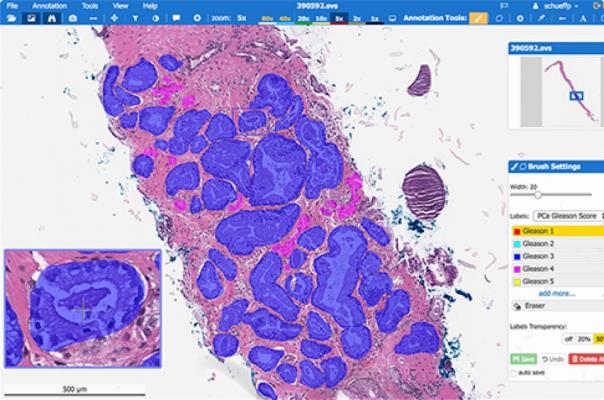
March 8, 2019 — Artificial intelligence (AI) startup company Paige.AI has been granted Breakthrough Device designation by the U.S. Food and Drug Administration (FDA) for its AI designed to enhance the clinical diagnosis and treatment of cancer. It is the first such designation for AI in cancer diagnosis publicly announced by any company, according to Paige.AI.
The FDA’s Breakthrough Device designation is granted for technologies that have the potential to provide for more effective diagnosis or treatment for life-threatening or irreversibly debilitating diseases. For these technologies, timely availability is in the best interest of patients because no approved alternative exists or because the technology offers significant advantages over existing approved alternatives. The Breakthrough Device program was created by the 21st Century Cures Act.
Paige.AI was launched in early 2018 based on technology developed by company co-founder Thomas Fuchs, Ph.D., and his colleagues and a license agreement with Memorial Sloan Kettering Cancer Center (MSK). MSK began digitizing its pathology slides four years ago. Under the license agreement, Paige.AI receives de-identified images of digitized slides – more than 1 million such slides to date — and is funding the digitization of an additional 4 million archive slides, which in total will create the largest digital pathology dataset. Paige.AI is working with this de-identified dataset to develop a comprehensive portfolio of AI products across cancer subtypes to serve the needs of pathologists around the world.
For more information: www.paige.ai


 February 06, 2026
February 06, 2026 









