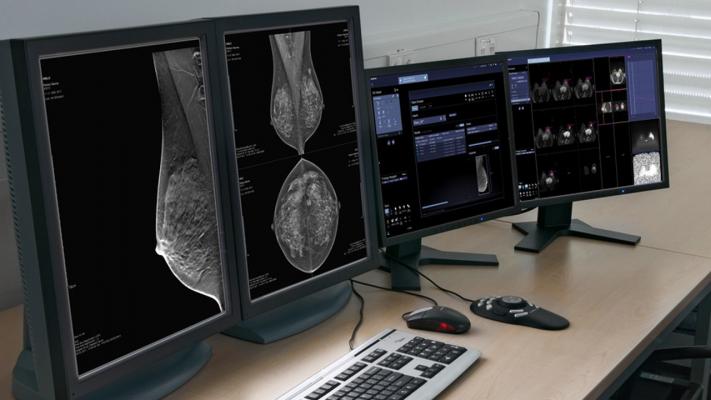
February 4, 2019 — Siemens Healthineers showcased the new planned artificial intelligence (AI)-based features with its mammography reading and reporting solution, syngo.Breast Care, at the 2018 Radiological Society of North America (RSNA) annual meeting, Nov. 25-30 in Chicago.1 These features are designed to provide physicians with interactive decision support.
AI features can help assist clinicians, particularly in cancer screening. A large number of mammograms are performed each day to screen for breast cancer, which means radiologists must accurately interpret hundreds of images daily. Additionally, the increased use of 3-D breast tomosynthesis adds to the number of images to be read. The new version of syngo.Breast Care is designed to give radiologists interactive clinical decision support.
AI-based algorithms are designed to help evaluate individual lesions and provide a system evaluation for 2-D mammograms or tomosynthesis. A peer-reviewed scientific study2 demonstrated increased sensitivity and specificity with the use of AI-based support.
Also, the planned software is designed to sort cases automatically and score those cases with a numerical value between 1 and 10. The case score is designed to take into account any existing lesions, microcalcifications and other abnormalities.
syngo.Breast Care’s planned new SmartSort technology is designed for radiologists to rank exams according to their preferences based on these case scores. For example, critical cases can be moved immediately to the top to receive priority.
Siemens Healthineers collaborated with ScreenPoint Medical and plans to integrate interactive decision support in syngo.Breast Care. The company’s mammography reading software, Transpara, is based on deep learning and has been trained with over 1 million images.
Transpara received FDA 510(k) clearance for 2-D reading in late November.
For more information: www.usa.siemens.com/healthcare
References
1. Syngo.Breast Care VB40 - powered by Transpara, ScreenPoint Medical - is currently under development. It is not for sale in the U.S. Its future availability cannot be guaranteed.
2 Rodriguez-Ruiz A., Gubern-Merida A., Lang K., et al.: Detecting breast cancer in mammography: a deep learning-based computer system versus 101 radiologists (RSNA abstract 2018).


 February 13, 2026
February 13, 2026 









