December 20, 2016 — The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance to Statlife’s DenSeeMammo, a software solution for breast density category assessment. DenSeeMammo provides a standardized and automatic breast density evaluation that mimics radiologists' visual assessment according to the BIRAD 5th Edition guidelines. The images from the mammography equipment are compared to a database of images that were previously quoted by a consensus of Mammography Quality Standards Act (MQSA) radiologists specialized in breast imaging.
The company said the software provides a breast density assessment in agreement with a radiologist's visual assessment, but in a very reproducible way. Only the breast density visually assessed by radiologists is recommended by the BIRAD Atlas V and is clinically significant considering the masking effect and the correlation with breast cancer risk.
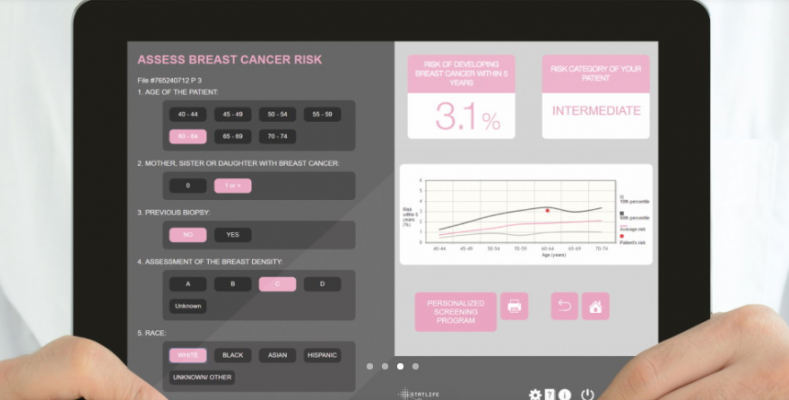
The DenSeeMammo software can be combined with Statlife’s MammoRisk software for breast cancer risk assessment, it provides a breast density category assessment, a risk evaluation, and a patient report with a personalized screening program.
DenSeeMammo is a software application intended for use with digital mammography systems. DenSeeMammo estimates BIRADs breast density value by analyzing digitally processed 2-D mammograms using a fully automated comparison procedure. It provides a BIRADs breast density 5th Edition category to aid radiologists in the assessment of breast density.
The software produces adjunctive information. It is not an interpretive or diagnostic aid when the final assessment of breast density category is made by an MQSA qualified interpreting physician.
DenSeeMammo is compatible with images obtained from GE Senographe Essentials systems.
For more information: www.mammorisk.com/us


 March 06, 2026
March 06, 2026 









