June 20, 2016 — Prostate cancer is the leading cancer among men, second only to skin cancer. With surgical removal at the frontline of defense, oncologists are considering prostate-specific molecular imaging at the point of initial biopsy and pre-operative planning to root out the full extent of disease, researchers revealed at the 2016 Annual Meeting of the Society of Nuclear Medicine and Molecular Imaging (SNMMI).
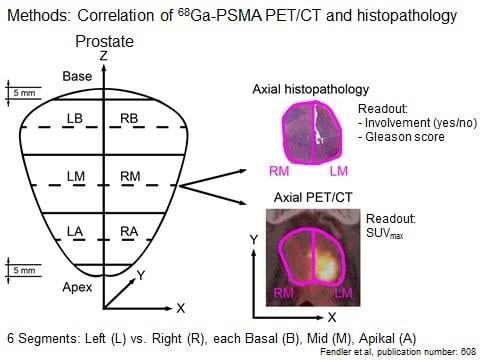
Positron emission tomography (PET) and computed tomography (CT) are often used in conjunction to image both the physiological function and structure of recurrent prostate cancer. In recent years, scientists have been developing a PET imaging agent that targets a protein called prostate-specific membrane antigen (PSMA). This protein is over-expressed on the surface of prostate cancer cells and can be detected even after they have spread to other organs. Researchers are able to detect prostate cancer by combining a small amount of radioactive material called gallium-68 and the molecular compound PSMA-HBED-CC (collectively known as Ga-68 PSMA), which is then injected prior to PET/CT scanning. Once administered, the agent binds to cells expressing PSMA and emits a signal detected by the scanner.
Scan images of the tumor data show active tumors glowing like hot coals where they are most virulent. While PSMA PET/CT is not yet the standard of image guidance for biopsies and surgical resection, the findings of this study show that this molecular imaging technique stands to improve tumor staging from the start.
“PSMA shows significant over-expression on prostatic cancer cells and Ga-68 PSMA PET/CT demonstrates a high rate of detection in patients with recurrent, metastatic prostate cancer. However much less research has been conducted for the accuracy of PSMA imaging at the start of the disease,” said Wolfgang P. Fendler, M.D., from the department of nuclear medicine at Ludwig-Maximilians-Universität of Munich in Munich, Germany. “The results of our study indicate that Ga-68 PSMA PET/CT accurately identifies affected regions of the prostate and might thus present a promising tool for non-invasive tumor characterization and biopsy guidance.”
Researchers evaluated subjects with histopathology and maximum standard uptake values using Ga-68 PSMA PET/CT to determine the boundaries of prostate tumor burden in the days prior to surgical resection. Results showed that the 67 percent of segmented tissues that tested positive for cancer via histological evaluation were positively identified by PET/CT via Ga-68 PSMA tumor uptake. With further investigation and regulatory approval, oncologists could one day use prostate-specific molecular imaging to aid needle biopsy and primary staging for better prostate-cancer patient care.
Approximately one out of seven men will be diagnosed with prostate cancer within their lifetime. About 180,890 new cases and 26,120 deaths from prostate cancer are estimated to occur in the U.S. alone in 2016, according to the American Cancer Society.
For more information: www.snmmi.org


 February 03, 2026
February 03, 2026 









