June 26, 2015 - New results in animals highlight a major safety concern regarding a class of magnetic resonance imaging (MRI) contrast agents used in millions of patients annually, according to a new paper. The paper was published online by the journal Investigative Radiology, which is published by Wolters Kluwer.
The study adds to concerns that repeated use of specific "linear"-type gadolinium-based contrast agents (GBCAs) lead to deposits of the heavy-metal element gadolinium in the brain. The results will have a major impact on the multimillion-dollar market for MRI contrast agents, predicts Investigative Radiology Editor-in-Chief Val M. Runge, M.D., of University Hospital Zurich. He commented, "This important safety issue may lead to certain linear GBCAs not being used in the future."
Led by Philippe Robert, Ph.D., of the French pharmaceutical company Guerbet, the researchers designed experiments in rats to assess the effects of repeated injections of GBCAs. These agents are widely used for diagnostic MRI scans, with approximately 30 million doses given each year worldwide.
Over five weeks, one group of rats received a series of 20 injections with gadodiamide, one of a class of agents known as "linear" GBCAs. Another group of animals were injected with a different type of GBCA-the "macrocyclic" agent gadoterate meglumine. (Robert's company, Guerbet, manufactures gadoterate meglumine.) A third group of rats received an inactive saline solution.
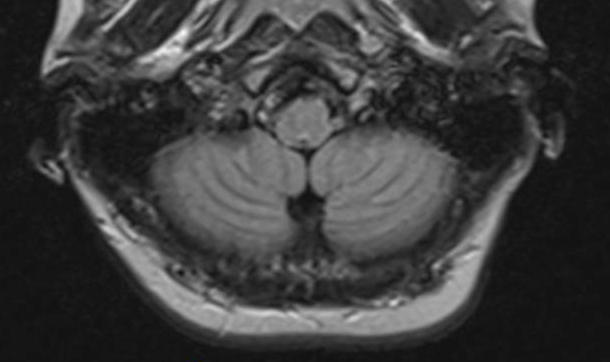
Over time, "significant and persistent" MRI abnormalities (called T1-weighted signal hyperintensities) developed in the brains of rats receiving the linear GBCA, gadodiamide. But no MRI abnormalities appeared in the brains of rats injected with the macrocyclic agent, gadoterate meglumine.
The increases in signal hyperintensity persisted even after the injections stopped. In subsequent examinations, high total gadolinium concentrations were measured in the deep brain (cerebellum) of gadodiamide-treated rats, corresponding to the area of the MRI abnormalities.
The findings are consistent with recent studies reporting T1 hyperintensities in human patients receiving multiple injections of linear GBCAs for MRI scans. "Certain of these agents lead to heavy-metal deposition in parts of the brain, which is not seen with the macrocyclic GBCAs," said Runge.
Gadolinium is the element employed as the basis of GBCAs, which have been widely used as MRI contrast agents for nearly three decades. "However, it is also a toxic heavy metal that is not a normal trace element in the body," Runge explained.
Certain linear GBCAs have previously been linked to a rare but serious disease called nephrogenic systemic fibrosis in patients with severely impaired kidney function. Macrocyclic GBCAs were originally developed as a safer alternative to linear GBCAs. Based on the new results, Runge predicts that certain GBCAs may soon fall out of use. A question raised by physicians is whether any patient, upon seeing this data, would want to be injected with the less stable agents.
In addition to the effects on patient care, the study may also have a major impact on the contrast agent industry. Runge believes that several products-accounting for a substantial part of annual sales worldwide-may be withdrawn. The estimated U.S. market for GBCAs is at least $300 million per year, about one-fourth of the world market.
The animal model developed for the study provides an important new scientific tool for evaluating which contrast media are associated with brain gadolinium deposits, and which are not, Runge believes. "All of the currently approved GBCAs should be evaluated by the methods used in the article by Robert et al, or by a similar approach," he wrote in an editorial accompanying the new paper. "This could lead, and if so appropriately, to the reassessment of the approval status of the least stable agents."
For more information: www.wolterskluwer.com


 January 28, 2026
January 28, 2026 









