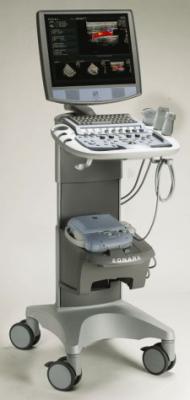
Ultrasound maker ZONARE Medical Systems showcased its new z.one ultra mobile ultrasound system, the company's next-generation Convertible Ultrasound platform, at the 2007 ASA meeting.
The company's proprietary Zone Sonography technology has enabled ZONARE to bring its patented Convertible Ultrasound platform to the industry. The company says it provides premium image quality and performance together with greater portability at lower price than most conventional ultrasound systems. Clinicians are able to convert the z.one system, at the touch of a button, from a full-featured, cart-based system into a premium compact ultrasound system, without sacrificing image quality or performance, the company said. The z.one ultra system also features advanced software, hardware and transducer technology, including a newly introduced basic cardiac package.


 February 05, 2026
February 05, 2026 









