July 8, 2009 - Ziosoft Inc., a leader in advanced visualization and analysis software for medical imaging, has received 510(k) clearance from the United States Food and Drug Administration (FDA) to market its magnetic resonance (MR) cardiac function analysis application for use with the Ziostation thin-client system.
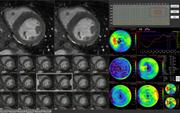
The MR cardiac function analysis application is used by physicians to evaluate the structures and function of the heart in the initial screening and subsequent management of coronary disease patients. Ziosoft’s MR cardiac function application provides physicians with the unique capability to extract left ventricle myocardial contours from Ziosoft's automatic interpolation algorithm to obtain highly accurate results.
In addition to the standard functional assessment calculations such as ejection fraction, peak filling rate (PFR) and peak ejection rate (PER), automatic calculation of myocardial mass is also generated with the Ziosoft application. The software provides the capability to automatically produce “bull’s eye” representations as well as a time volume graph to aid in the patient evaluation. Ziosoft’s application has the ability to display different sequences in one view, allowing clinicians to scroll between sequences and positions simultaneously. This exclusive feature provides an intuitive method of displaying patient information for more proficient image interpretation. The MR cardiac function application can be accessed throughout the enterprise on the Ziostation thin-client system to provide physicians with improved efficiency and real-time collaboration when assessing results from an MR study.
For more information: www.ziosoftinc.com


 April 18, 2025
April 18, 2025 









