December 13, 2011 — At RSNA 2011, Ziltron will launch its Web-based Perform & Director Product Suite to measure, analyze and improve the diagnostic capability, quality and performance of radiology professionals. The company, based in San Diego, Calif. with a European base in Limerick, Ireland, delivers observer performance and digital imaging diagnostics.
Traditionally, performance-testing and quality assurance for the radiology industry has lacked standards-based technology and analytics to gauge the proficiency of reading examinations among radiologists. This has been true both for training and in the field; accuracy of test and examinations has been a subjective exercise until now.
The company’s suite of training, educational and accreditation solutions significantly improves digital imaging diagnostic accuracy. Combining high-quality image viewing, real-time data capture and reporting facilities, the technology delivers accurate statistical feedback on performance. The company’s tools are ideal for accreditation, quality assurance, certification and CME programs.
One of Ziltron’s early customers is BreastScreen Australia (BSA), which aims to reduce morbidity and mortality from breast cancer in Australia by the early detection of breast cancer. The BreastScreen Reader Assessment Strategy (BREAST) is a resource by which BreastScreen Australia may continue to meet these aims.
The strategy is based on digital screen reading test sets by Ziltron designed to assess performance of screen readers and provide immediate feedback to individual readers and BreastScreen Services.
“Ziltron has the proven expertise and knowledge to support national and international bodies to transform the education and performance of breast imaging,” said Patrick Brennan, professor of medical imaging, University of Sydney, and project manager for Breast Australia. “Their tools are effective, modern and adaptive and should suit the needs of clinicians and scientists in a variety of domains.”
Ziltron’s assets include:
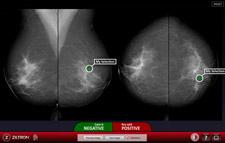
· Diagnostic assessment: The company’s digital imaging diagnostics suite allows users to diagnose confirmed cases; the technology then measures the ability of the user to make a correct diagnosis, and compare that diagnosis to the universe of others who have taken the same test.
· User observation: Statistical algorithms measure all elements of the user experience including detailed data on the user’s interaction with the system during the test process. The system monitors and measures Graphical User Interface (GUI) tools such as zooming, contract/brightness control and image navigation; it also records the duration of individual case selections and the overall test set. This detailed data enables administrators and researchers to evaluate granular user observation patterns that can be used in future tests and improve the test sets and diagnostic competence of users.
· Advanced statistical analysis: Data is processed in real time, and users can receive a comprehensive set of statistical results upon completion of the test set. Results are based on statistical analysis important to digital imaging diagnosis including: receiver operating characteristic (ROC), sensitivity, specificity, location sensitivity, and true/false positives and negatives. Groups, institutions and peer-to-peer analysis and reports are available; performance comparisons are also available in graphical format using the “Heat Map” applications.
The Perform & Director Product Suite is a performance, quality assurance and educational tool to significantly improve the knowledge of professionals whose area of expertise involves digital imaging diagnosis. The product is available in myriad formats including web-based, PC and Mac applications, iPad and smartphones.
Ziltron will display in booth #1108 at RSNA 2011.
For more information: www.ziltron.com


 February 11, 2026
February 11, 2026