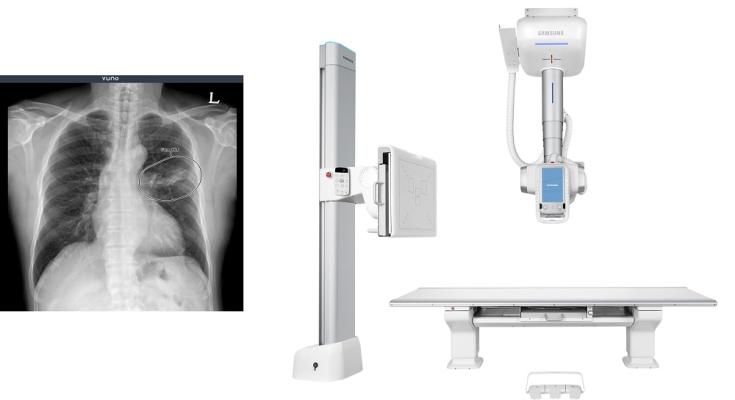
October 19, 2021 — South Korean artificial intelligence (AI) developer, VUNO Inc., announced that the company closed a deal with global medical device manufacturer, Samsung Electronics Co., Ltd., to embed VUNO’s artificial intelligence-driven chest X-ray diagnostic solution, VUNO Med-Chest X-ray into Samsung’s GC85A premium ceiling type digital radiography system.
The latest contract follows an earlier deal in June with Samsung to supply the VUNO Med-Chest X-ray solution to the electronics maker’s mobile digital radiography system GM85, making the AI solution an adjunct to Samsung’s mobile and ceiling type digital X-ray systems and available for use by customers in Korea as well as Europe and other key markets around the world.
VUNO Med-Chest X-ray is an AI-empowered medical device that assists clinicians in the detection of areas that are suspicious for pulmonary diseases such as tuberculosis and pneumonia by accurately identifying thoracic abnormalities on chest radiographs. Its lightweight feature driven by the optimization of AI calculation algorithm is the key factor that enables seamless integration with a wide range of X-ray devices, delivering AI results on the spot upon scanning an image.
Hyun-jun Kim, co-founder and CEO of VUNO said that “thanks to the solution’s excellent capabilities as well as a proven track record in a wide range of X-ray devices under various clinical settings, we were able to sign another deal to include VUNO Med-Chest X-ray in the two premium digital X-ray systems of Samsung Electronics”. He added, “VUNO will continue to work closely with global medical device manufactures including Samsung Electronics to further expand our presence in the world market.”
Woo-young Jang, Samsung Electronics’ Head of DR Business Team said, “This deal will allow Samsung Electronics to provide an advanced system that integrates AI technology into our key premium X-ray devices to markets world-wide.”
For more information: www.vuno.co


 February 06, 2026
February 06, 2026 









