January 28, 2013 — Varian Medical Systems has received a U.S. Food and Drug Administration (FDA)- 510(k) clearance for the latest version of its Vitesse real time planning for HDR brachytherapy which is used to plan and perform high-dose-rate (HDR), ultrasound-guided brachytherapy treatments for prostate cancer.
"This version of Vitesse reduces the number and complexity of steps involved in planning and completing a treatment. It eliminates the need for a data transfer to another software program, and avoids moving the patient to a CT scanner for images in the middle of the procedure. For these reasons, most clinicians will see a reduction in the amount of time needed to complete these treatments, often by as much as an hour and a half," said Tim Clark, marketing manager for Varian BrachyTherapy.
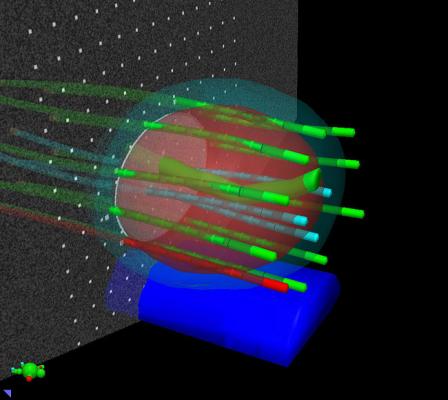
HDR brachytherapy involves delivering radiotherapy from inside the body by temporarily placing a tiny radioactive source directly into the tumor or other targeted area. Using a robotic device called an afterloader, clinicians place the radioactive source into positions through needles that have been inserted into the area being treated. The source is then moved within the needles under computer control to create the specified dose distribution within the patient's anatomy.
Using the latest version of Vitesse, HDR brachytherapy treatment plans can be created in a real-time environment, using ultrasound images generated in the operating room rather than computed tomography (CT) scans generated elsewhere. This avoids the need to move the patient to a CT scanner room for imaging after the needles have been put in place. The process can now be completed entirely within the Vitesse program, from capturing the ultrasound image to finalizing an approved treatment plan.
In addition to receiving FDA 510(k) clearance, which permits sale of the Vitesse software in the United States, Varian has also obtained the CE mark which permits sales in the European Union and other countries where the CE mark is recognized.
For more information: www.varian.com


 July 11, 2024
July 11, 2024