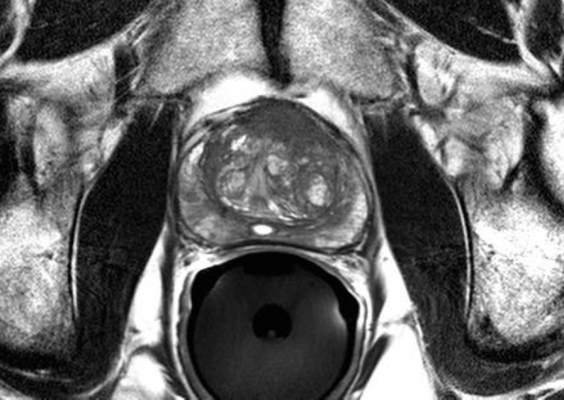
February 8, 2021 — The results of a Phase III randomized clinical trial have shown that when it comes to detecting clinically significant prostate cancer, magnetic resonance imaging (MRI) with targeted biopsies (MRI-TBx) matches the current standard and brings a multitude of advantages. The PRostate Evaluation for Clinically Important Disease: MRI vs Standard Evaluation Procedures (PRECISE) study will help to make prostate cancer diagnosis more accurate and less invasive.
PRECISE included 453 participants at Canadian academic cancer centres who were either assigned to receive MRI imaging followed by MRI-TBx of suspicious areas (identified by MRI), or the current standard of care of a systematic 12-core transrectal ultrasound-guided (TRUS) biopsy (TRUS-Bx).
Key findings:
- MRI with targeted biopsy found five per cent more clinically significant prostate cancers compared to those receiving systematic TRUS-Bx biopsies, conclusively demonstrating the method can at least match the performance of the current standard of care.
- Compared to standard TRUS-Bx, the MRI-TBx were found to be better in identifying clinically significant cancers.
- More than a third of patients in the MRI arm of the trial avoided biopsies altogether following negative imaging results. Those individuals received a follow-up MRI in two years' time.
- Those who did have biopsies in the MRI arm had significantly fewer samples taken when compared to systematic TRUS-Bx, resulting in less pain and discomfort for patients. Moreover, the MRI arm had a decreased adverse event profile, including less hematuria (blood in the urine) and incontinence.
- There is a major unmet need for a test that identifies clinically significant prostate cancer while avoiding overdiagnosing clinically insignificant cancers. Use of MRI reduced the unnecessary diagnosis of slow growing, clinically insignificant prostate cancers by 55 per cent.
These findings show decisively that MRI together with targeted biopsies offer patients a less invasive procedure, the chance to avoid a biopsy all together and can help avoid the over-treatment of clinically insignificant prostate cancer - all while detecting a higher rate of clinically significant cancers.
"My colleagues and I are thrilled about these results that show, without a doubt, that imaging and targeted biopsies are the future of prostate cancer diagnosis. We can catch more of the cancers we should be treating, avoid unnecessary treatment at the same time and improve the quality of life for our patients." said Laurence Klotz, M.D., Chair of Prostate Cancer Research at Sunnybrook Health Sciences Centre and lead author of the study. "We thank the study participants and our funders for their support and look forward to continuing our efforts to have this technology used more widely."
"The study's findings have influenced Ontario Health-Cancer Care Ontario's upcoming, updated Prostate MRI Guidelines, which will be released this year," said Masoom Haider, M.D., co-lead of the study and Professor of Medical Imaging at the University of Toronto, and Clinician Scientist with the Ontario Institute for Cancer Research. "I am pleased to see our research produce results that will make a real difference in how prostate cancer is diagnosed and improve the lives of patients."
"I congratulate Dr. Klotz and the PRECISE team on this truly impactful research which will change clinical care and make a difference for men with prostate cancer," said Christine Williams, M.D., Deputy Director and Head, Clinical Translation at the Ontario Institute for Cancer Research. "It is a great example of how, with our partners, we are moving research innovations to the clinic to improve the lives of patients and treat cancer with improved precision."
"These practice-changing results will have a significant and positive impact on the roughly 64 Canadians who are diagnosed with prostate cancer every day. Thanks to the efforts of Dr. Klotz and his team, people will need to undergo fewer biopsies and for some of them, they will be spared from unnecessary biopsies and treatments altogether," said Stuart Edmonds, M.D., Executive Vice President, Mission, Research and Advocacy at the Canadian Cancer Society. "We are proud to support this research, which will help people with prostate cancer live longer, fuller lives."
"At Movember, we are honoured to play a role in funding cutting-edge research like the PRECISE study, ultimately helping to provide more positive outcomes for men living with or beyond a prostate cancer diagnosis," said Todd Minerson, Country Director for Movember Canada.
For more information: www.


 February 13, 2026
February 13, 2026 









