Patients answered three questionnaires (Quick-Disabilities of the Arm, Shoulder, and Hand [QDASH] and two parts of the Boston Carpal Tunnel Syndrome Questionnaire: symptom severity [BCTSQ-SS] and functional status [BCTSQ-FS] scales) assessing the affected wrist's function and discomfort immediately pre-procedure, 2 weeks post-procedure, and at least one year post-procedure. Infographic courtesy of the American Roentgen Ray Society (ARRS), American Journal of Roentgenology (AJR)
September 17, 2020 — According to ARRS' American Journal of Roentgenology (AJR), ultrasound-guided carpal tunnel release (UGCTR) quickly improves hand function and reduces hand discomfort, making UGCTR a safe, effective, and less invasive alternative to traditional open or endoscopic surgery.
Because ultrasound guidance allows carpal tunnel release to be performed with smaller incisions and quicker recovery, five researchers from Thomas Jefferson University Hospital in Philadelphia, Pa., set out to evaluate UGCTR's long-term efficacy in improving function and discomfort in patients with carpal tunnel syndrome.
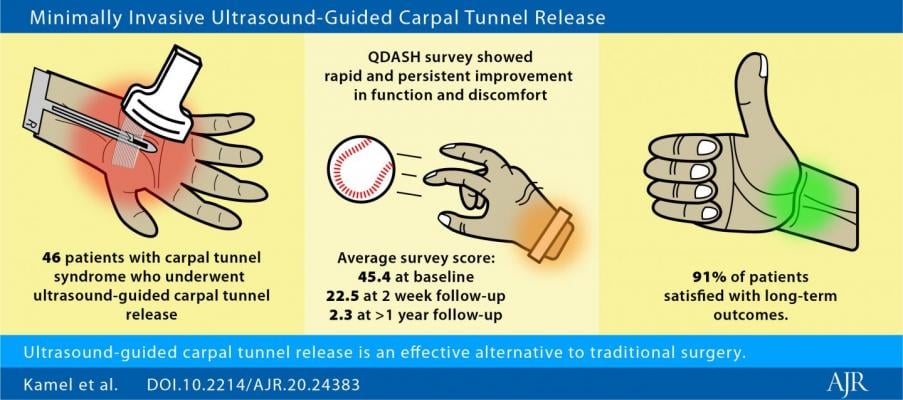
Sixty-one UGCTR procedures performed in 46 patients (25 women and 21 men; mean age 60.6 years) with clinically diagnosed carpal tunnel syndrome were retrospectively reviewed. All procedures were performed under local anesthetic at an outpatient radiology office using the SX-One MicroKnife (Sonex Health).
As first AJR author Sarah I. Kamel explained: "Patients answered three questionnaires (Quick-Disabilities of the Arm, Shoulder, and Hand [QDASH] and two parts of the Boston Carpal Tunnel Syndrome Questionnaire: symptom severity [BCTSQ-SS] and functional status [BCTSQ-FS] scales) assessing the affected wrist's function and discomfort immediately pre-procedure, 2 weeks post-procedure, and at least one year post-procedure."
Median pre-procedure scores were 45.4 for QDASH, 3.2 for BCTSQ-SS, and 2.5 for BCTSQ-FS. Median 2 week post-procedure scores were 22.5 for QDASH, 1.7 for BCTSQ-SS, and 1.9 for BCTSQ-FS--all decreased (p < 0.001) from pre-procedure scores and surpassing reference standards for clinically important difference in scores.
Follow-up questionnaires were obtained for 90% (55/61) of wrists, a median of 1.7 years post-procedure, with further declines (p < 0.001) in median scores: 2.3 for QDASH, 1.2 for BCTSQ-SS, and 1.1 for BCTSQ-FS. At long-term follow-up, 96% (52/54) of wrists demonstrated lower QDASH, and 98% (53/54) lower BCTSQ (average of BCTSQ-SS and BCTSQ-FS), vs pre-procedure scores.
Although no immediate postoperative complications occurred, the authors of this AJR article detailed several response modifications for two patients who required surgery for complications experienced 8-10 days postoperatively (one for infection following injury and one for post-traumatic compartment syndrome).
"The procedure now includes more extensive preprocedural cleaning that extends to the forearm circumferentially prior to draping. A Tegaderm (3M Company, St. Paul, MN) is now placed at the distal third of the forearm to act as an additional sterile barrier at the edge of the sterile field. In addition, two passes of the ligament transection are performed routinely on all patients to potentially decrease the risk of remnant tissue that may contribute to incomplete release," Kamel et al. added.
For more information: www.arrs.org


 February 16, 2026
February 16, 2026 









