July 29, 2021 — Exo (pronounced “echo”), a pioneering health information and devices company, closed a $220 million Series C investment round led by RA Capital Management and joined by BlackRock, Sands Capital, Avidity Partners and Pura Vida Investments that will fund the commercialization of its handheld ultrasound device and its intuitive point-of-care ultrasound workflow solution, Exo Works. Prior investors also participated.
Developed by a team of nanotechnologists, chip designers, consumer electronics and medical device experts, Exo’s handheld ultrasound device will, once regulatory approvals have been obtained, use radically new materials, processes and AI in an effort to generate unrivaled image quality, definition and depth at an affordable cost. This scalable technology will allow Exo to widely distribute a device that aims to decentralize ultrasound while making it accessible everywhere.
"Exo has developed a transformative point-of-care ultrasound solution. Their best-in-class imaging coupled with a simple and intuitive workflow will make them a leader in this exciting field. We have been thoroughly impressed by the strong and growing team at Exo. We are excited to work alongside them as they rapidly expand access to this powerful technology," said Zach Scheiner, principal with RA Capital Management.
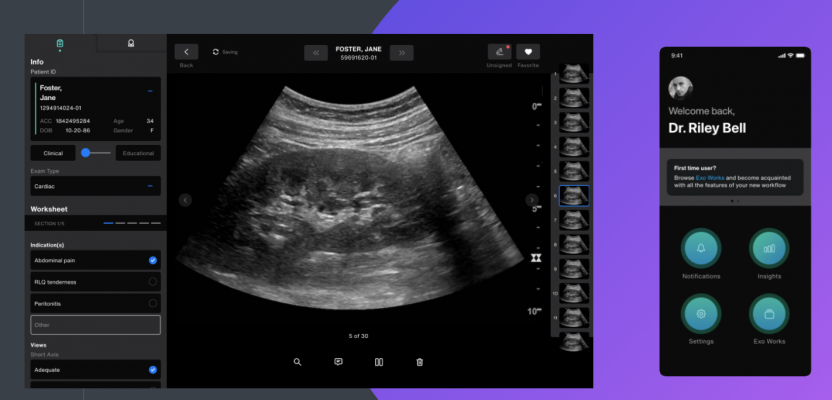
Exo is a pioneer in ultrasound silicon technology, which is the foundation of its handheld ultrasound device that can be used for the entire body. Meticulously engineered to work within a chaotic emergency department, rural clinic or across multiple departments in a community hospital, the powerful handheld ultrasound device will be simple and intuitive to operate.
Exo’s medical imaging ecosystem includes its proprietary point-of-care ultrasound workflow solution, Exo Works. It solves decades-long workflow issues by streamlining exam review, documentation and billing in one platform — in under 60 seconds.The software works with nearly all point-of-care ultrasound devices and securely connects to the most common EMR and PACS systems used in hospitals to house imaging and communications. The ease of connectivity finally makes interoperability a reality and disconnected ultrasound processes a thing of the past.
The combination of Exo’s handheld ultrasound device and Exo Works will seamlessly blend an entire medical imaging ecosystem to perform flawlessly in one of the most demanding real-world applications of technology, while unlocking numerous applications across a wide swath of medical care. Uses such as predictive diagnostics could influence patients to make better choices and adopt healthier lifestyles prior to a confirmed diagnosis. Remote quality assurance and credentialing could allow experienced providers at different institutions to review and approve medical imaging. Ultrasound scans via telemedicine would enable patients at home to be sent a probe and guided through a scan by an experienced provider.
“Exo’s hardware and software were designed in tandem, with the future of decentralized healthcare at the forefront of every decision,” said Exo CEO Sandeep Akkaraju. “Our vision is a healthcare system unconstrained by the four walls of a hospital and engineered for a world where providers can see clearly into every patient immediately.”
This wave of medical innovation will decentralize complex and cumbersome healthcare processes, ushering in a new era of medical care. With powerful mobile technology in the hands of physicians at the point-of-care, layers of complexity, minutes of lost time and inter-department inefficiencies are all stripped from the patient care process. Meanwhile small clinics, rural hospitals and international health organizations are all empowered to provide next-generation diagnostics immediately and effectively.
“Handheld ultrasound is changing how medical care is delivered, which has already been proven by its use during the COVID-19 pandemic when it was difficult to bring cart-based systems into triage areas,” said Arun Nagdev, MD, director of emergency ultrasound at Highland General Hospital and senior director of clinical education at Exo. “Emergency medicine will become so much more precise, swift, patient-focused and outcome-oriented as physicians are educated and empowered by intuitive handheld ultrasound devices that are at the ready for everything from traumatic injury diagnosis to nerve-block procedures.”
Whether pinpointing veins for IVs, evaluating cardiological conditions or scanning the lungs of COVID-19 patients, Exo’s powerful device will aim to see deeper and provide more accurate imaging for a wide range of body types, including obese and overweight patients that are hard to scan effectively with current handheld ultrasound technology.
The team at Exo is made up of executives with deep ultrasound experience, MEMS expertise and consumer device development. Vice President of Marketing Jeff Peiffer and Vice President of Sales Andy Berthusen, who will lead Exo’s commercialization efforts, have a combined 45 years of experience leading global product development, marketing and sales at GE Healthcare and Johnson & Johnson.
The Series C funding follows a successful Series B funding round for the Redwood City-based company, bringing Exo’s total funding to more than $320 million. As Exo accelerates commercialization of both its hardware and software, the company is proud to have the backing of a world-class syndicate of healthcare and health technology investors.
Morgan Stanley & Co LLC served as sole placement agent for the transaction. Wilson Sonsini Goodrich & Rosati served as legal advisor to Exo.
For more information: www.exo.inc


 February 16, 2026
February 16, 2026 









