November 26, 2023 — TeleDaaS, PLLC, a leading dosimetry-as-a-service provider, made its official debut today at the Radiological Society of North America’s annual meeting, #RSNA2023. TeleDaaS specializes in delivering clinical-grade, precision-based dosimetry analysis and treatment plans to clinical research organizations (CROs) and pharmaceutical manufacturers. TeleDaaS is designed to provide highly-scalable, best-in-class dosimetry services that integrate with existing imaging workflows, to transform cancer treatment research and development.
"We are launching TeleDaaS today to arm those researching and developing breakthrough cancer treatments with the technology and services needed to usher in the next generation of precision cancer therapies,” said TeleDaaS Chief Medical Officer Dr. Mark Crockett, M.D. “Radiopharmaceuticals are revolutionizing modern cancer treatment and precision dosimetry is a crucial component of this advanced therapy. By marrying proven technology and expertise in personalized dosimetry, coupled with the accessibility and scalability of telemedicine, TeleDaaS is committed to pushing the boundaries of what is possible in this field."
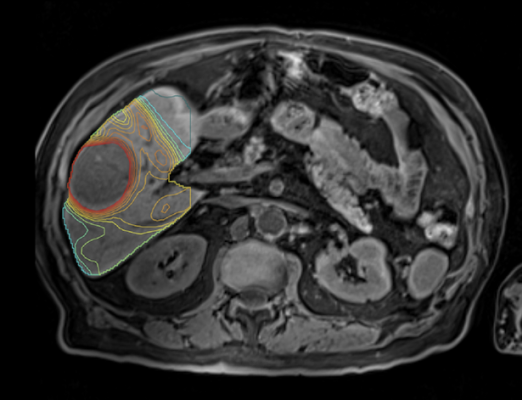
At TeleDaaS, a team of leading dosimetry practitioners leverage proprietary software from Mirada Medical, LTD, which integrates with standard imaging technology, to support clinical research organizations in clinical trial design, execution and the development of new targeted radiation therapies. TeleDaaS’s platform is highly scalable for clinical trials and offers CROs and pharmaceutical companies flexibility with regard to hiring in-demand dosimetrists.
“We are seeing exciting developments in the field of dosimetry to keep up with the rapid expansion to different radioisotopes and corresponding dosimetric requirements,” says Dr. Jessica Guarnaschelli, MD. “I anticipate that the benefit of radiopharmaceuticals will be maximized by combining radiopharmaceutical therapies with a vast array of other agents including immunotherapies, chemotherapeutics, radiosensitizers and radioprotectors. This is already showing promise with several ongoing trials of approved and developmental radiopharmaceuticals. With the advancements in this field, TeleDaaS enters the market at a crucial time when the science and the technology align.”
TeleDaaS’s technology and processes are tightly governed by robust quality methodologies and the platforms are information security compliant. TeleDaaS licenses a cloud-based technology platform from Mirada Medical. Through a service agreement with Mirada, TeleDaaS leverages the ISO 13485 and ISO 27001 compliance maintained by Mirada Medical.
Mirada’s personalized, or multi-compartment, dosimetry technology has been used in multiple clinical trials to dramatically improve clinical outcomes, including DOSISPHERE-1 which compared personalized dosimetry with standard dosimetry. The life expectancy of patients on the personalized dosimetry arm of the trial increased to 26.6 months from 10.7 on the standard dosimetry arm of the trial. The benefits of personalized dosimetry were so stark that the trial was cut short because it was deemed unethical to assign patients to the standard dosimetry arm of the trial.
RSNA 2023 attendees can visit TeleDaaS in the North Hall, level 3 at booth #7952.
For more information: https://www.teledaas.com/


 February 04, 2026
February 04, 2026 









