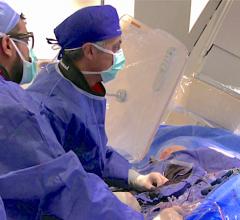
December 7, 2009 - A new integrated hybrid interventional table offers head-to-toe tilting and side-to-side cradling to meet the needs of both interventionalists and surgeons during hybrid intervention. Because patient access is critical for physicians performing endovascular, open surgical or hybrid cath lab procedures, the CAT-880B hybrid catheterization table is designed to create a hybrid interventional suite that deploys Toshiba’s Infinix-i five-axis X-ray systems. “Using this new hybrid catheterization table in conjunction with Toshiba’s bi-plane five-axis cath lab is the final piece in creating a premium hybrid suite,” said John Cheatham, M.D., director, cardiac catheterization and interventional therapy. “The table is uniquely designed to meet the individual needs of both interventionalists and surgeons. It allows them to perform the same functions used in their respective home environments – the operating room and the cath lab – together in a single hybrid suite without any sacrifice in patient access, ergonomics or efficiency.” When working on patients in a hybrid setting, it is critical the imaging system provides outstanding image quality, as well as the flexibility to reach ancillary equipment and the patient quickly and easily. The new hybrid catheterization table features a 550-pound table weight limit, making this table ideal for a range of patients, from pediatric to bariatric. It also allows for angulations of up to 16 degrees in all four directions and offers low table-top height for the catheterization table. The 75-cm table height is particularly important for open surgical procedures, as it provides patient access and physician comfort, regardless of the procedure being performed. With the new table, Toshiba’s Infinix-i five-axis X-ray systems are designed to accommodate endovascular catheter-based techniques, open surgical settings or a combined hybrid approach, for procedures, including hypoplastic left heart syndrome and intraoperative stent therapy. Both Dr. Cheatham, M.D. and Mark Galantowicz, M.D., chief, cardiothoracic surgery, both of The Heart Center of Nationwide Children's Hospital, are recognized for pioneering the hybrid approach to treat congenital heart conditions. They worked with Toshiba engineers to design the new table and evaluated the prototype to ensure it met their needs in a real-life hybrid setting. Today, Nationwide Children’s is the first site with this hybrid catheterization table in use at its hybrid cath lab. The table is currently available on all five-axis Infinix-i systems and will be available for other Infinix-i configurations in the near future. Toshiba America Medical Systems, Inc. has introduced the new hybrid catheterization table at this year’s Radiological Society of North America (RSNA) annual meeting, held in Chicago, Nov. 29 – Dec. 4, 2009. For more information: www.medical.toshiba.com


 August 14, 2025
August 14, 2025