December 17, 2009 - Partial breast irradiation "is an approach that may allow more patients to undergo breast-conserving therapy more quickly, at lower cost, and with less risk of long-term complications,” said The American College of Surgeons (ACS) in a consensus statement.*
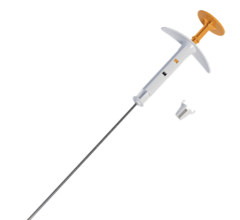
In the document, the ACS emphasized the advantages of accelerated partial breast irradiation by delivering brachytherapy with open single-entry devices with multiple lumens. This technique includes SAVI radiation therapy provided at the Breast Clinic of Memphis. SAVI provides APBI, a shortened course of high-dose radiation therapy for early-stage breast cancer patients following lumpectomy surgery. The treatment is completed in just five days — compared to the six weeks of treatment, five days a week, required for traditional, external-beam radiation.
The device's multi-catheter design allows physicians to target radiation to the area that needs it most, minimizing exposure to healthy tissue. Precisely sculpted radiation is delivered from within the breast and targets the area where the cancer is most likely to recur.
In related news, Cianna Medical announced it has received ISO 13485:2003 certification for its quality management system for the design, development and manufacture of the SAVI breast brachytherapy applicator, which delivers targeted radiation therapy for the treatment of early-stage breast cancer.
The certification allows Cianna Medical to continue with the CE Mark registration process, which is required for sales within the European Economic Community. The CE Mark will apply to all four sizes of SAVI and the SAVI Prep Catheter.
The device's multi-catheter design enables physicians to direct radiation where it is needed most, while minimizing exposure to healthy structures, like the skin, chest wall or lungs. The ability to customize radiation based on patient-specific anatomy is said to help reduce complications typically associated with radiation therapy and allows physicians to offer the five-day treatment to more women.
The SAVI applicator received its 510(K) clearance from the U.S. Food & Drug Administration in July 2006.
*The ACS statement, titled “Image-Detected Breast Cancer: State-of-the-Art Diagnosis and Treatment,” was published in the October 2009 issue of the Journal of the American College of Surgeons.
For more information: www.facs.org and www.ciannamedical.com


 October 01, 2023
October 01, 2023