December 5, 2018 — Subtle Medical announced 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market SubtlePET. Subtle Medical also recently secured approval to affix the CE Mark on SubtlePET to begin marketing in the European Economic Area without restrictions.
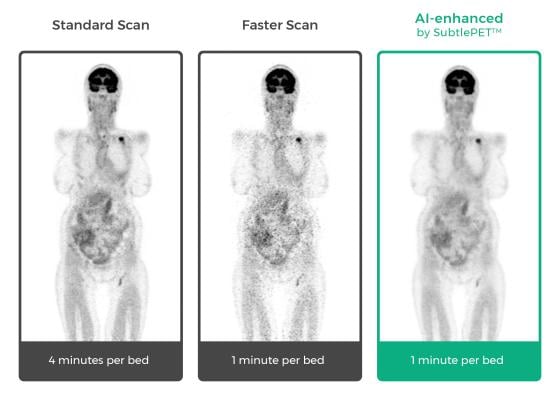
SubtlePET’s artificial intelligence (AI)-powered technology allows hospitals and imaging centers to enhance images from faster positron emission tomography (PET) scans, leading to an improved patient experience during nuclear imaging procedures, while boosting exam throughput and provider profitability. SubtlePET is currently in pilot clinical use in multiple university hospitals and imaging centers in the U.S. and abroad.
“Focusing Subtle Medical's SubtlePET AI platform on faster image acquisition, we have been able to dramatically increase PET scan efficiency and provide a superior patient experience. SubtlePET technology allows us to scan a patient four times faster than normal, yet maintain equal image quality, not otherwise impacting work flow,” said Michael Brant-Zawadzki, M.D., FACR, Hoag Hospital, Newport Beach, Calif. “This creates immediate ROI [return on investment] benefit for our hospital and a compelling value proposition. I’m looking forward to seeing more groundbreaking technology from the Subtle team.”
Subtle Medical’s AI solution:
- Enables completion of more exams in a day compared to conventional PET imaging without the need for capital expenditures;
- Reduces patient time in the scanner; and
- Helps hospitals and imaging centers enhance their bottom line in today’s competitive healthcare environment.
The company’s technology utilizes deep learning algorithms that integrate seamlessly with any OEM scanner and picture archiving and communication system (PACS) to enhance images during acquisition, without any interruption or alteration in the imaging specialists’ workflow. SubtlePET delivers a significant improvement in the image quality of noisy images resulting from shorter scans, which is particularly beneficial for children and those undergoing repeat PET exams.
SubtlePET is the first product in Subtle Medical’s growing portfolio of new AI technologies to receive FDA clearance. The company is developing additional products to be submitted for FDA clearance. A second product currently undergoing clinical evaluation is SubtleMR, which allows imaging centers to significantly accelerate magnetic resonance imaging (MRI) scans using the company’s AI solutions. SubtleGAD is also being developed to reduce gadolinium dosage during imaging procedures.
For more information: www.subtlemedical.com


 February 06, 2026
February 06, 2026 









