April 7, 2014 — Cianna Medical announced the results of a retrospective study of breast cancer patients with ductal carcinoma in situ (DCIS) treated with SAVI breast brachytherapy. Researchers reported excellent local control, with recurrence rates that appear to be equivalent to those expected for whole breast irradiation (WBI) with similar follow-up.
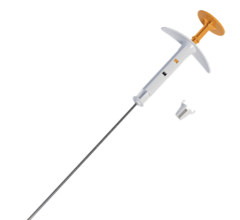
SAVI is a strut-based device that delivers a form of accelerated partial breast irradiation (APBI) known as breast brachytherapy, a five-day course of targeted radiation for early-stage breast cancer. With more than 10 years of clinical evidence showing excellent outcomes in efficacy and safety, breast brachytherapy is proven to be an appropriate treatment for early-stage breast cancer.
“This study adds to the growing body of research showing that brachytherapy-based APBI is a safe and effective treatment for DCIS patients,” said lead author John Einck, MD, a radiation oncologist at the University of California San Diego Moores Cancer Center. “With very low rates of recurrence that compare favorably to the data for whole breast irradiation, our data supports the use of brachytherapy in DCIS patients across a broad range of ages.”
The study reported on 248 DCIS patients treated from 2007 to 2011, making it the largest study presented to date on the use of strut-based breast brachytherapy to treat DCIS. For the entire cohort, there were five ipsilateral breast recurrences (1.75 percent recurrence rate).
Researchers stratified patients by age at the time of treatment completion (?60 yrs, ?50-<60 yrs and <50 yrs) and reported local recurrence rates for each group, which were 1.26 percent, 1.10 percent and 5.56 percent, respectively. Median follow-up was greater than two years for all three groups.
Data came from the SAVI Collaborative Research Group (SCRG), which studies the long-term outcomes of women treated with strut-based brachytherapy, and was presented at the annual meeting of the American Brachytherapy Society, April 3-5.
Similarly favorable recurrence rates with strut-based brachytherapy have been reported with even greater long-term follow-up. Data from the SCRG presented at the American Society for Radiation Oncology (ASTRO) meeting in September 2013 reported “excellent local control” with a 2 percent recurrence rate at four-and-a-half years median follow-up.
“APBI eases the burden of a breast cancer diagnosis by reducing treatment time without compromising recurrence risk,” said Catheryn Yashar, M.D., also a radiation oncologist at the UC San Diego Moores Cancer Center and co-principal investigator of the SCRG. “It’s a proven treatment for a certain group of patients, and this is yet another study that shows we can safely offer this accelerated treatment to an even larger group of women.”
Strut-based brachytherapy is used as part of breast conservation therapy (BCT), which consists of a lumpectomy followed by radiation therapy. Traditionally, the radiation portion consisted of WBI, which requires six to seven weeks of daily treatments. Breast brachytherapy delivers radiation to the area immediately surrounding the lumpectomy cavity, sparing healthy tissue from unnecessary radiation and enabling treatment to be completed in as little as five days. Patient eligibility for breast brachytherapy is based on a number of factors, including tumor size, type of cancer, age, margin status and lymph node involvement.
The strut-based, open architecture design of SAVI allows physicians to sculpt radiation based on patient-specific anatomy, which may also increase the number of women eligible for breast brachytherapy.
For more information: www.ciannamedical.com


 October 01, 2023
October 01, 2023