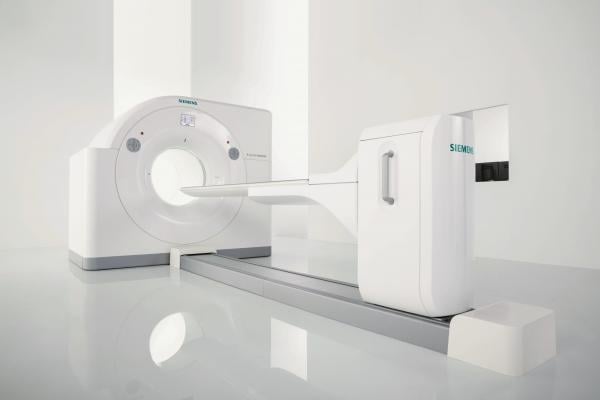
January 21, 2016 — Siemens Healthcare has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the Biograph Horizon positron emission tomography/computed tomography (PET/CT) system, which offers premium performance at a low total cost of ownership. The system leverages proven PET/CT technology to help providers address more clinical indications in oncology, neurology and cardiology, while also introducing new efficiencies and cost savings.
Biograph Horizon enables physicians to visualize small lesions early. The system’s 4 mm LSO crystals scintillate faster and have a higher light output than BGO crystals, resulting in high resolution and better image quality, and enabling Time-of-Flight acquisition. Biograph Horizon’s capabilities are valuable for many indications, like lung cancer – the most common PET/CT indication, at 29 percent of the total exam mix. As more than half of lung cancer patients are diagnosed at a late stage and have an increased likelihood of distant metastasis, more detailed patient data can help clinicians more accurately stage disease and evaluate therapy response, contributing to more effective care pathways.
The recent decision of the U.S. Preventive Services Task Force (USPSTF) to recommend annual lung cancer screening with low-dose CT for high-risk adults has the potential to generate more suspicious findings, for which biopsies are often performed to confirm malignancy. However, 43 percent of these biopsies come back negative, and 1 in 6 result in an adverse event such as lung collapse, which can cost up to 28 times more than the cost of incorporating PET/CT into the evaluation following lung cancer screening. High-contrast, high-resolution PET/CT with Time-of-Flight can help clinicians identify additional diagnostic procedures, like biopsy, that are most appropriate for each lung cancer patient, supporting improved patient outcomes, lower procedure costs and reduced liability risks. To help providers address the growing demand for CT lung exams, Biograph Horizon also can be used as a shared service to support CT overflow, reduce patient wait times and enable scheduling flexibility.
As the smallest PET/CT system with the lowest power requirements, Biograph Horizon minimizes the initial capital investment and is a smart choice for institutions looking to replace their current PET/CT scanner, according to Siemens, as the system will fit into virtually any existing PET/CT exam room. Biograph Horizon offers low operating and maintenance costs to help manage the total cost of ownership, with automated technologies such as gentle system warm-up and automatic standby that extend the system’s economic life in addition to reducing power consumption. And Siemens’ Guardian Program predicts system issues before they occur, lowering costs related to unplanned downtime by 43 percent.
To increase productivity and time spent with patients, Biograph Horizon simplifies staff’s daily routine by automating manual tasks and offering protocol-based exams. For example, the Quanti•QC feature can run quality control procedures overnight, scans can be performed in as fast as 5 minutes, and reconstruction runs alongside acquisition for image delivery just 30 seconds after the scan.
Configured specifically for Biograph Horizon, syngo.via Molecular Imaging Workplace is a cost-effective image processing solution that can expand with providers’ clinical needs. The solution offers automated tools to instantly visualize diagnostic information, measure with confidence and report more comprehensively. syngo.via automates prefetching, preprocessing, and display and comparison of previous findings for up to 45 percent faster processing. Also, ALPHA technology provides automatic registration with organ-based recognition capabilities. EQ•PET normalizes standard uptake value (SUV) measurements across different scanners for more precise calculation of changes in tumor uptake.
For more information: www.usa.siemens.com


 February 27, 2026
February 27, 2026 









