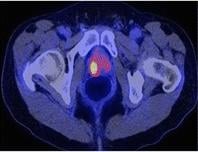
June 24, 2015 - Siemens' PETNET Solutions, a wholly owned subsidiary of Siemens Healthcare, announced it will provide clinical researchers in the greater New York City area with access to gallium-68-labeled prostate-specific membrane antigen (Ga-68 labeled PSMA). This investigational tracer is being studied for prostate cancer imaging using positron emission tomography/computed tomography (PET/CT) in clinical trials throughout the world.
Prostate cancer is the second most frequently diagnosed cancer among men in the United States. An estimated 220,000 men are expected to be diagnosed with the disease this year, with approximately 15,000 in New York state alone. Recent hypotheses regarding the variation and diversity of prostate cancer suggest that all variants should not be treated equally – underscoring a need for expanded classification of the disease through imaging and biopsy methods.
For more information: www.siemens.com


 February 03, 2026
February 03, 2026 









