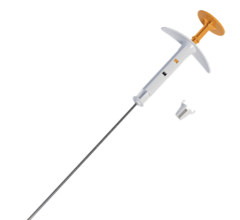
May 26, 2009 - SenoRx will deliver three oral presentations summarizing clinical findings related to its Contura Multi-Lumen Balloon (MLB) Catheter at the American Brachytherapy Society (ABS) Annual Meeting being held May 31-June 2, 2009, in Toronto, Canada.
"The three scheduled presentations, along with other studies, continue to support our belief that a patient treated with Contura MLB can receive a more targeted dose of radiation, while minimizing radiation to healthy tissue such as the skin and ribs," said SenoRx President and CEO Lloyd Malchow.
Presentations to be delivered at the ABS conference are: "Initial Dosimetric Experience: Contura Multi-Lumen Balloon (MLB) Registry Trial," by Arthur, D.W.; Vicini, F.A.; Todor, D.; and Julian, T.B. The conclusion of this study is that without the dosimetric optimizing capabilities, the safe use of a single lumen balloon is limited to patients with ideal cavity geometry and balloon symmetry. This initial comparison shows that the use of multiple, off-set lumens allow the optimization of target coverage while minimizing dose to nearby skin and chest wall, thus creating a new improved dosimetric standard.
The other study is: "A Comparison of Skin and Chest Wall Dose Delivered with Multi-Catheter (MC), Contura Multi-Lumen Balloon (MLB), and MammoSite (MS) Breast Brachytherapy" by Cuttino, L.W.; Todor, D.T.; Heffernan, J.; Vera, R.; and Arthur, D.W. The conclusion of this study is that the multicatheter and Contura MLB techniques are associated with significantly lower mean skin and chest wall doses than with MammoSite, which was associated with significantly more patients receiving doses to these areas in excess of 125 percent of the prescription dose. Doses delivered with Contura MLB were more similar to those of the multi-catheter technique, which is associated with a very low incidence of skin and chest wall toxicity, suggesting treatment with Contura MLB may prove to be better tolerated.
The third study is titled, "Verification of Balloon Rotation for the Contura Multi-Lumen Device for Accelerated Partial Breast Irradiation," by Ouhib Z., Benda, R., Kasper, M., Vargas, C. This study’s conclusion says that the alignment of the skin mark with lumen number one can be a reliable tool to align the balloon prior to treatment and also that no internal device rotation or torquing is likely to occur. This is critical for the MLB as there may be significant variability in dwell times between catheters and various dwell points within a catheter to allow for skin and/or rib sparing while maintaining adequate dose coverage.
For more information: www.senorx.com


 October 01, 2023
October 01, 2023